Oleanolic Acid Alleviates Hyperuricemia via Gut Microbiota Control the Integrity of Gut Barrier and the Expressions of Urate Transporter in Mice
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Hyperuricemia (HUA) is a globally prevalent metabolic disorder characterized by an imbalance in uric acid (UA) production and excretion. In this study, we found that oleanolic acid (OA), a natural pentacyclic triterpene, effectively reduced HUA and associated kidney injury in C57BL/6J mice. A 12-week OA treatment significantly and dose-dependently reduced UA and creatinine levels in serum and urine while suppressing hepatic xanthine oxidase activity in HUA mice. Mechanistic analysis revealed that OA modulates the expression of urate transporters including ABCG2, GLUT9, and URAT1 in the kidney and small intestine. Furthermore, OA restored gut microbiota imbalances, increased short-chain fatty acid production, and enhanced the expressions of intestinal tight junction proteins in HUA mice, thereby improving gut barrier integrity in HUA mice. Consequently, fecal microbiota transplantation (FMT) was employed to illustrate the major mediating role of gut microbiota in OA’s alleviation of HUA in mice. Recipient HUA mice transplanted with feces from OA-treated HUA mice exhibited significantly lower blood and urinary UA levels, reduced kidney inflammation, and improved gut microbiota balance compared to those receiving feces from untreated HUA mice (p < 0.05). Additionally, FMT normalized urate transporter expression and reinforced intestinal tight junctions in recipient mice. These findings underscore that OA mitigates HUA primarily by modulating gut microbiota, regulating urate transporter expression, and reinforcing gut barrier integrity, offering novel insights into its preventive potential for managing HUA and related complications.
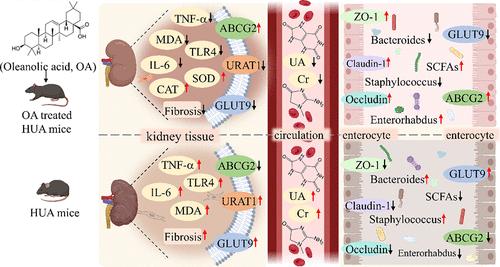
齐墩果酸通过肠道菌群控制肠道屏障完整性和尿酸转运蛋白表达减轻小鼠高尿酸血症
高尿酸血症(HUA)是一种全球普遍存在的代谢性疾病,其特征是尿酸(UA)的产生和排泄不平衡。在本研究中,我们发现齐墩果酸(OA)是一种天然的五环三萜,可以有效地减少C57BL/6J小鼠的HUA和相关的肾损伤。12周的OA治疗显著且剂量依赖性地降低了HUA小鼠血清和尿液中的UA和肌酐水平,同时抑制了肝黄嘌呤氧化酶活性。机制分析显示,OA可调节肾和小肠中尿酸转运蛋白ABCG2、GLUT9和URAT1的表达。此外,OA恢复了HUA小鼠肠道菌群失衡,增加了短链脂肪酸的产生,增强了肠道紧密连接蛋白的表达,从而改善了HUA小鼠肠道屏障的完整性。因此,粪便微生物群移植(FMT)被用来说明肠道微生物群在OA减轻小鼠HUA中的主要介导作用。与接受未经处理的HUA小鼠粪便的小鼠相比,接受oa处理的HUA小鼠的粪便移植的受体HUA小鼠表现出显著降低的血液和尿UA水平,减轻肾脏炎症,改善肠道微生物群平衡(p <;0.05)。此外,FMT使受体小鼠的尿酸转运蛋白表达正常化,并增强了肠道紧密连接。这些发现强调,OA主要通过调节肠道微生物群、调节尿酸转运蛋白表达和加强肠道屏障完整性来减轻HUA,为其预防HUA和相关并发症的潜力提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


