Temperature-orientation changes in ROS-oxidized egg white protein conformation modulate the thermal aggregation behavior
IF 8.5
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
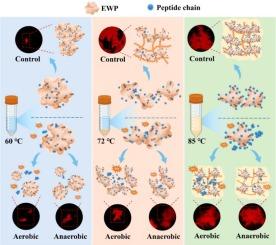
In this paper, the differences in thermal aggregation behavior of egg white protein (EWP) mediated by reactive oxygen species (ROS) at different heat temperatures were investigated. Results showed that an increase in EWP turbidity and the change in particle size during the heating process depended on the interactions after protein peptide chain unfolding. With the increase in heating temperature, the EWP aggregates changed from indeterminate fiber-like structure to regular network structure. The thermal stability results showed an increase in the thermal stability of EWP after oxidation. The formation of thermally induced aggregates was accompanied by a significant increase in the hydrophobicity of the protein surface from 249.93 to 2748.10. Raman spectroscopy indicated that oxidized EWP exposed hydrophobic groups to inhibit aggregation during heating, and EWP demonstrated significant anti-aggregation properties when heated at 72 °C. This study provides certain theoretical support for improving the thermal processing level of egg products.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


