Amyloid fibrils from black kidney bean protein self-assemble into hydrogels: Impact of heating time on gel structure and rheological properties
IF 8.5
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
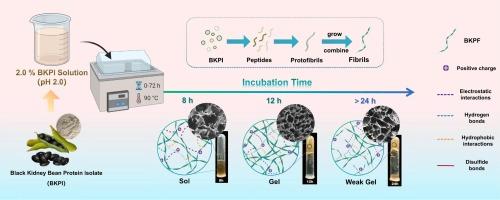
Fibrillation of plant proteins is a promising approach to enhance their gel properties. In this study, black kidney bean protein isolate self-assembled into amyloid fibrils and subsequently formed hydrogels at a concentration of 2.0 wt% after thermal treatment at pH 2.0. Gel structure and rheological properties were modulated by regulating the acid-heat incubation time (0–72 h). With increased incubation time, the black kidney bean protein fibrils (BKPFs) transitioned through distinct states: sol state (8 h), gel state formed by fibril entanglement (12−20 h), and disrupted gel state by partial depolymerization of fibril aggregates (>24 h). Rheological analysis revealed that the gels at 16 h had maximum storage modulus (159.9 Pa). Small-angle X-ray scattering indicated that BKPFs highly aggregated (Rg = 49.49 nm) with a more compact mass fractal structure (Df = 2.0) at 16 h. Cryo-scanning electron microscopy images showed the formation of a homogeneous and dense three-dimensional gel network structure at 12 h.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


