Analysis of the mechanism of difference in umami peptides from oysters (Crassostrea ariakensis) prepared by trypsin hydrolysis and boiling through hydrogen bond interactions
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
This study compares umami peptides prepared by trypsin hydrolysis and boiling and analyzes their umami intensity and characteristics. Using a taste reconstitution model and taste evaluation analysis, the study revealed that umami peptides prepared by boiling have a higher umami contribution. Myosin and heat shock protein were identified as marker proteins for revealing differences of cleavage sites. Boiling releases a higher proportion of acidic amino acids at the protein cleavage sites p1-p1’, whereas trypsin hydrolysis releases more basic amino acids. Molecular docking simulation and electrostatic potential observation showed that acidic amino acid residues have a wider binding range with the umami receptor T1R1-VFT. Acidic amino acids lower the isoelectric point (pI) of umami peptides, enhancing their negative charge at pH 7, which are more likely to bind to the positively charged regions of the umami receptor.
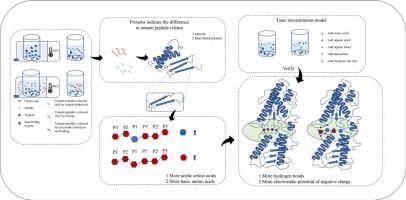
胰蛋白酶水解和氢键相互作用沸煮牡蛎鲜味肽差异的机理分析
本研究比较了胰蛋白酶水解法和水煮法制备的鲜味肽,分析了它们的鲜味强度和鲜味特性。通过味觉重建模型和味觉评价分析,研究表明,通过煮沸法制备的鲜味肽具有较高的鲜味贡献。肌凝蛋白和热休克蛋白被确定为揭示裂解位点差异的标记蛋白。沸煮在蛋白质裂解位点p1-p1 '释放更多的酸性氨基酸,而胰蛋白酶水解释放更多的碱性氨基酸。分子对接模拟和静电电位观察表明,酸性氨基酸残基与鲜味受体T1R1-VFT的结合范围更广。酸性氨基酸降低鲜味肽的等电点(pI),增强其在pH 7时的负电荷,从而更有可能与鲜味受体的正电荷区域结合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


