SIGNIFICANT IMPACT OF CONSUMABLE MATERIAL AND BUFFER COMPOSITION FOR LOW-CELL NUMBER PROTEOMIC SAMPLE PREPARATION
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
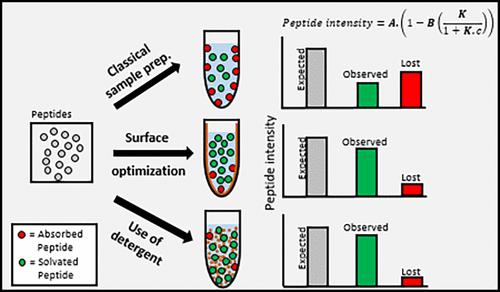
Proteomics, essential for understanding gene and cell functions, faces challenges with peptide loss due to adsorption onto vial surfaces, especially in samples with low peptide quantities. Using HeLa tryptic digested standard solutions, we demonstrate preferential adsorption of peptides, particularly hydrophobic ones, onto polypropylene (PP) vials, leading to nonuniform signal loss. This phenomenon can alter protein quantification (e.g., Label-Free Quantification, LFQ) if no appropriate data processing is applied. Our study is based on understanding this adsorption phenomenon to establish recommendations for minimizing peptide loss. To address this issue, we evaluated the nature of surface material and buffer additives to reduce peptide-surface noncovalent binding. Here, we report that using vials made from polymer containing polar monomeric units such as poly(methyl methacrylate) (PMMA) or polyethylene terephthalate (PET) drastically reduces the hydrophobic peptide loss, increasing the global proteomics performance (4-fold increase in identified peptides for the single-cell equivalent peptide content range). Additionally, the incorporation of nonionic detergents like poly(ethylene oxide) (PEO) and n-Dodecyl-Beta-Maltoside (DDM) at optimized concentrations (0.0001% and 0.0075%, respectively) improves the overall proteomic performance and consistency, even across different vial materials. Implementing these recommendations on 0.2 ng/μL HeLa tryptic digest results in a 10-fold increase in terms of peptide signal. Application to True Single-Cell sample preparation without specialized instrumentation dramatically improves the performance, allowing for the identification of approximately 650 proteins, a stark contrast to none detected with classical protocols.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


