Activator Strand Modifications in CRISPR/Cas12a: Unlocking the Potential for Casp-3-Targeted Biosensing and Imaging Analysis of Apoptosis
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
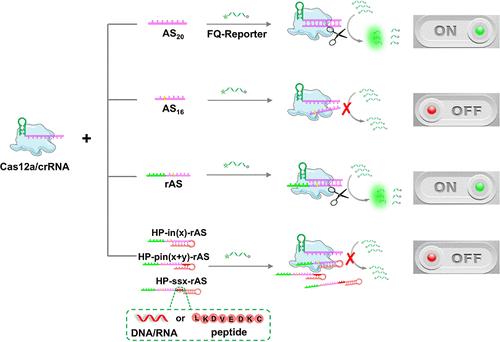
The CRISPR/Cas12a system has emerged as a powerful tool in biosensing due to its unique trans-cleavage activity. This study conducted an in-depth investigation of the modulatory capabilities of this system, particularly focusing on the 5′-end modifications of the activator strand, and found that introducing a hairpin structure (HP) at the 5′-end of the activator strand, which was designed based on the RESET effect, can effectively suppress the activator strand’s ability to activate the trans-cleavage activity of the CRISPR/Cas12a system. This suppression is independent of the HP’s relation to the activator strand and the type of linker used (DNA, RNA or peptide). Detaching the HP from the activator strand restores the system’s activity. These findings enrich the development of CRISPR/Cas12a-based biosensors, and expand their application beyond DNA-based target detection to peptide sequence-based target recognition. Based on this discovery, we constructed a sensitive biosensor for caspase-3 (Casp-3), a key executor in apoptosis, by linking the HP to the activator strand with a peptide linker containing a Casp-3 recognition site. The proposed biosensor has been validated for its sensitivity and specificity in detecting Casp-3, as well as for monitoring drug-induced apoptosis through the imaging of Casp-3 in living cells, providing a valuable tool for studying the apoptotic process, screening drugs, assessing drug efficacy, and evaluating treatment outcomes. This strategy also shows promise for detecting other peptide-based targets, broadening the horizons for early disease biomarker detection and timely therapeutic interventions.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


