Competitive binding of flavors in the preparation of soy protein: Screening based on molecular docking
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Competitive binding of flavor compounds to proteins offers a strategy for mitigating off-flavors in soy protein isolate (SPI). We hypothesized that highly competitive pleasant flavor compounds can facilitate the release of off-flavors by occupying their binding sites during SPI preparation. Molecular docking identified four binding pockets on β-conglycinin (7S) and glycinin (11S) proteins interacting with off-flavors. From 54 pleasant flavors, 30 were screened, focusing on seven: δ-nonalactone, δ-decalactone, δ-undecalactone, furaneol, geraniol, nerol, and geranyl acetate. Sensory evaluation and gas chromatography–mass spectrometry (GC–MS) analysis demonstrated that these compounds significantly reduced the intensity of unpleasant flavor attributes and decreased off-flavor content under high concentration neutral conditions (10 % w/v, pH 7.0) and low concentration acidic conditions (3 % w/v, pH 4.0). Structural differences influenced binding efficacy, with shorter-chain compounds like furaneol outperforming long-chain lactones. These findings provide a novel strategy for off-flavor removal in SPI, supporting the development of consumer-preferred soy products.
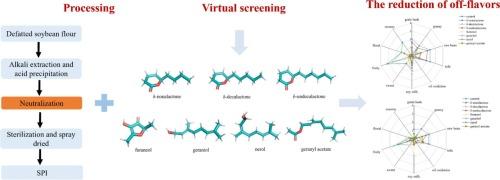
大豆蛋白制备中风味的竞争结合:基于分子对接的筛选
风味化合物与蛋白质的竞争性结合为减轻大豆分离蛋白(SPI)中的异味提供了一种策略。我们假设,在SPI制备过程中,高度竞争的令人愉悦的风味化合物可以通过占据它们的结合位点来促进异味的释放。分子对接鉴定出β-甘氨酸(7S)和甘氨酸(11S)蛋白与异味相互作用的四个结合袋。从54种令人愉悦的香料中筛选出30种,重点是δ-非内酯、δ-癸内酯、δ-癸内酯、呋喃醇、香叶醇、橙花醇和香叶乙酸酯7种。感官评价和气相色谱-质谱(GC-MS)分析表明,在高浓度中性条件(10 % w/v, pH 7.0)和低浓度酸性条件(3 % w/v, pH 4.0)下,这些化合物显著降低了不愉快风味属性的强度,降低了异味含量。结构差异影响结合效果,短链化合物如呋喃醇优于长链内酯。这些发现为去除SPI中的异味提供了一种新的策略,支持了消费者偏好豆制品的开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


