Efficient Escherichia coli Platform for Cannabinoid Precursor Olivetolic Acid Biosynthesis from Inexpensive Inputs
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
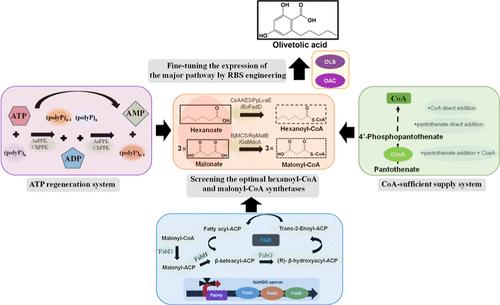
Olivetolic acid (OLA), an initial precursor of cannabinoids, is catalyzed by type III polyketide synthase, which has a wide range of pharmacological activities, such as antimicrobial and cytotoxic effects. Here, we applied systematic metabolic engineering to develop a multienzyme cascade system to produce OLA via two low-cost inputs. The polyketide synthase (OLS) and cyclase enzymes (OAC), along with the best combination of hexanoyl-CoA and malonyl-CoA synthetases (AEE3 and MatB), were first introduced into the biocatalytic system to increase the supply of hexanoyl-CoA and malonyl-CoA as starting and extender units. To drive the catalysis smoothly, an ATP regeneration system and a CoA-sufficient supply system were incorporated into the biocatalysts to provide enough cofactors. Furthermore, malonyl-CoA flux was redirected to OLA biosynthesis through delicate control of the fatty acid biosynthesis (FAB) pathway via promoter engineering. Collectively, these strategies have led us to produce OLA at a titer of 102.1 mg/L with a productivity of 25.5 mg/L/h by using malonate and hexanoate as direct substrates. Our biocatalytic system provides an effective platform for the production of the cannabinoid precursor OLA in Escherichia coli and may be a valuable reference for the development of microbial cell factories that use hexanoyl-CoA and malonyl-CoA as important intermediates.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


