Highly Efficient Biosynthesis of Rebaudioside M9 through Enzyme Screening and Structure-Guided Engineering
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
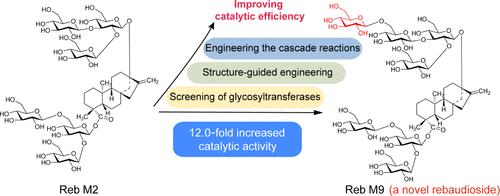
Steviol glycosides (SGs) are highly valued for their sweetness, safety, and zero calories, but their bitter taste and low solubility limit their application. Modifying glycosyl units is a promising strategy to improve sensory qualities. In this study, we identified the enzyme UGT94E13 through phylogenetic analysis and enzyme screening, which catalyzes the glycosylation of rebaudioside M2 (Reb M2) at the C-13 position, producing the novel β-1,6-O-glycosylated product rebaudioside M9 (Reb M9). Subsequently, the catalytic activity of UGT94E13 toward Reb M2 was enhanced 12.0-fold through a structure-guided enzyme engineering strategy, and the mechanism behind this enhancement was analyzed. Finally, an enzymatic cascade system comprising the optimal mutant UGT94E13-F169A/I185A and sucrose synthase AtSuSy was constructed and optimized, achieving efficient synthesis of Reb M9 with a yield of 98.3% and a titer of 42.8 g·L–1. Overall, this study provides an effective method for enhancing glycosylation of SGs and a reference for the glycosylation modification of natural products.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


