Mapping of Amyloid-β Aggregates In Vivo by a Fluorescent Probe with Dual Recognition Moieties
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
The spontaneous aggregation of amyloid-β (Aβ) leads to neuronal cell death in the brain and causes the development of Alzheimer’s disease (AD). The efficient detection of the aggregation state of Aβ holds significant promise for the early diagnosis and subsequent treatment of this neurodegenerative disorder. Currently, most of the fluorescent probes used for the detection of Aβ fibrils share similar recognition moieties, such as the N,N-dimethylamino group, N,N-diethylamino group, and piperidyl group. The development of fluorescent probes incorporating novel recognition groups will, in principle, bring new properties for the detection and imaging of Aβ aggregates. Herein, we designed and synthesized three fluorescent probes (1 CarbCN, 2 NTCN, and 3 NCarbCN) based on dicyanoisophorone. The probe 3 NCarbCN, which integrated two recognition moieties, a carbamate unit (a new recognition group) together with a N,N-dimethylamino group, showed a sensitive turn-on fluorescence response toward the early aggregation state of Aβ, along with a high binding affinity (Kd = 59 nM) and recognition selectivity for Aβ fibrils. Theoretical calculations revealed that the carbamate unit of 3 NCarbCN could provide an additional three hydrogen bonding interaction with Aβ42 fibrils. Furthermore, the probe 3 NCarbCN efficiently crossed the blood–brain barrier and exhibited a higher response in the brains of AD model mice. Co-staining of isolated brain sections with monoclonal antibody further demonstrated specific binding of 3 NCarbCN to Aβ plaques in the brains of AD model mice, thus demonstrating its great potential for the early diagnosis of such neurodegenerative disorders.
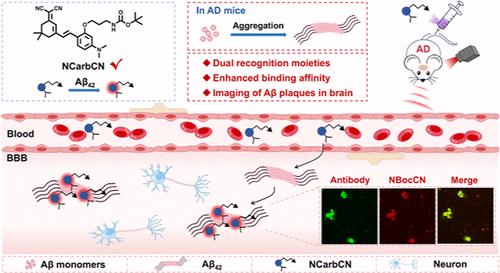
利用具有双重识别分子的荧光探针绘制体内淀粉样蛋白-β聚集体图谱
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


