Validating Centralized Biobanking Workflows for NMR Metabolomics Using the PRIMA Panel
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
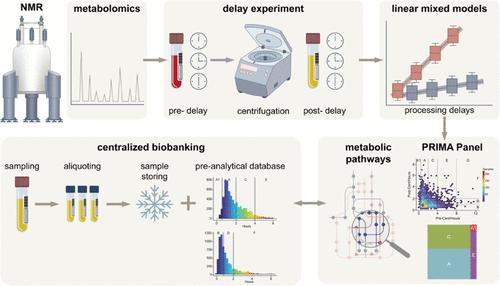
The quality of biological samples used in metabolomics research is significantly influenced by preanalytical factors, such as the timing of centrifugation and freezing. This study aimed to evaluate how preanalytical factors, like delays in centrifugation and freezing, affect metabolomics research. Blood samples, collected in various tube types, were subjected to controlled pre- and postcentrifugation delays. Metabolite levels were quantified using NMR spectroscopy and fitted in linear mixed models used to predict changes in metabolite concentrations over time. The results showed that some metabolites, such as lactic acid, were significantly affected by even short delays, while others remained stable for longer. The study introduced the concept of a “stability time point”, marking when a metabolite’s concentration changes by 20%. These predictive models were validated in a separate cohort. To apply these findings, the authors developed the PRIMA Panel, an open-source R Shiny tool. This tool allows researchers to assess the impact of preanalytical variations on their samples, predict metabolite stability, and generate performance reports. The PRIMA Panel was tested using samples from the Dresden Integrated Liquid Biobank, proving its utility in a real-world biobank setting. The study emphasizes the importance of tracking preanalytical factors to improve the reliability of metabolomics analyses. The PRIMA Panel is available online and for local deployment, providing a practical solution for quality control in metabolomics research. The results of the study underscore the importance of tracking preanalytical factors in biobanking. A versatile tool for assessing their impact on metabolic data is introduced, improving the reliability of future analyses.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


