Discovery of an Enzyme-Activated Fluorogenic Probe for In Vivo Profiling of Acylaminoacyl-Peptide Hydrolase
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
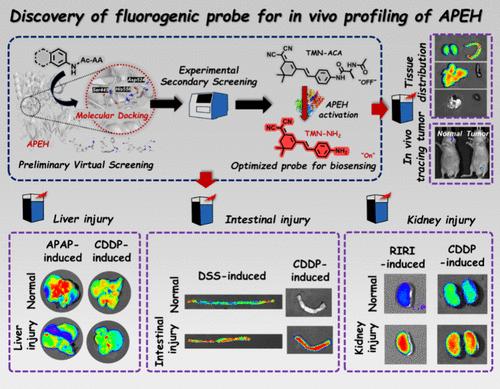
Acylaminoacyl-peptide hydrolase (APEH), a serine peptidase that belongs to the prolyl oligopeptidase (POP) family, catalyzes removal of N-terminal acetylated amino acid residues from peptides. As a key regulator of protein N-terminal acetylation, APEH was involved in many important physiological processes while its aberrant expression was correlated with progression of various diseases such as inflammation, diabetics, Alzheimer’s disease (AD), and cancers. However, while emerging attention has been attracted in APEH-related disease diagnosis and drug discovery, the mechanisms behind APEH and related disease progression are still unclear; thus, further investigating the physiological role and function of APEH is of great importance. To date, enzyme-activated fluorescent probes targeting POPs have been extensively reported and adopted in relevant medical research and applications. Nevertheless, as an important member of the POP family, APEH was rarely referred in the field of bioimaging while the fluorescent probe for in vivo sensing of APEH activity has not been reported yet. Thus, acquiring an efficient APEH-targeted probe is in urgent need. Herein, an enzyme-activated fluorogenic probe for in vivo profiling of APEH was first discovered via a substrate mimic-based strategy. By combination of stimulated molecular docking-based preliminary screening and experiment-based secondary screening, the optimal probe (named as TMN-AcA), which displayed high binding affinity, sensitivity, and specificity toward APEH, was screened out. Owing to the superior properties of TMN-AcA, endogenous APEH activity in various cell lines and transplanted tumor could be visualized while tissue distribution of APEH was revealed. Most importantly, APEH was first demonstrated to be a potential biomarker of multiple-organ injury via TMN-AcA-based bioimaging and immunohistochemistry (IHC) analysis while the newly developed probe could serve as a vital tool for APEH-related disease diagnosis and biological function study.
一种酶激活荧光探针在体内分析酰基氨基酰基肽水解酶
酰基氨基酰基肽水解酶(APEH)是一种丝氨酸肽酶,属于脯氨酸寡肽酶(POP)家族,催化肽中n端乙酰化氨基酸残基的去除。作为蛋白n端乙酰化的关键调控因子,APEH参与了许多重要的生理过程,其异常表达与炎症、糖尿病、阿尔茨海默病(AD)、癌症等多种疾病的进展相关。然而,尽管APEH相关疾病的诊断和药物发现引起了人们的关注,但APEH及其相关疾病进展的机制仍不清楚;因此,进一步研究APEH的生理作用和功能具有重要意义。迄今为止,针对持久性有机污染物的酶激活荧光探针已被广泛报道并应用于相关的医学研究和应用。然而,作为POP家族的重要成员,APEH在生物成像领域很少被提及,用于体内检测APEH活性的荧光探针尚未见报道。因此,迫切需要获得一种高效的针对apeh的探针。本文首先通过底物模拟策略发现了用于APEH体内分析的酶激活荧光探针。通过基于受激分子对接的初步筛选和基于实验的二次筛选相结合,筛选出对APEH具有高结合亲和力、敏感性和特异性的最佳探针TMN-AcA。由于TMN-AcA的优越性质,可以可视化各种细胞系和移植肿瘤中内源性APEH的活性,同时揭示APEH的组织分布。最重要的是,通过基于tmn - aca的生物成像和免疫组织化学(IHC)分析,APEH首次被证明是多器官损伤的潜在生物标志物,而新开发的探针可以作为APEH相关疾病诊断和生物学功能研究的重要工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


