Stepwise Lighting Up Gold(I)–Thiolate Complexes from AIE Nanoaggregates to AIEE Nanoprobes with a ZIF-8 Shell for Glucose Biosensing
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Aggregation-induced emission (AIE) or aggregation-induced emission enhancement (AIEE) has endowed gold species with responsive fluorescent properties, favoring their potential applications in sensing, imaging, and therapy. However, it remains an interesting challenge to fabricate fluorophores with both AIE and AIEE effects. Herein, we presented highly luminescent Au(I) thiolate nanocomplex-based biosensors with Zn2+ induced-AIE and zeolite imidazolate framework (ZIF-8) induced-AIEE effects. The nonemissive monovalent gold–glutathione complexes (AuI-SGs) were obtained to synthesize the core–shell Zn2+/AuI-SG@ZIF-8 composites with strong luminescence via the coordination-assisted self-assembly strategy. By immobilizing GOx on the surface of Zn2+/AuI-SG@ZIF-8, Zn2+/AuI-SG@ZIF-8/GOx biosensors exhibited effective responsiveness to glucose, showing a “turn-off” detection model. The mechanism study revealed that the robust luminescence of Zn2+/AuI-SG@ZIF-8 to glucose sensing was attributed to the acid-stimulated degradation of the probe facilitated by H+ generated from the glucose oxidase (GOx)-catalyzed oxidation process. To achieve noninvasive and intelligent blood glucose detection, the Zn2+/AuI-SG@ZIF-8/GOx-loaded microneedle (MN)-patch fluorescent platform was further developed. The MN-patch-based sensing platform had promising performance for on-needle capture and in situ glucose detection. This study demonstrated a universal and feasible protocol to construct luminescent biosensors for glucose detection and their potential for the development of MN-based analytical devices.
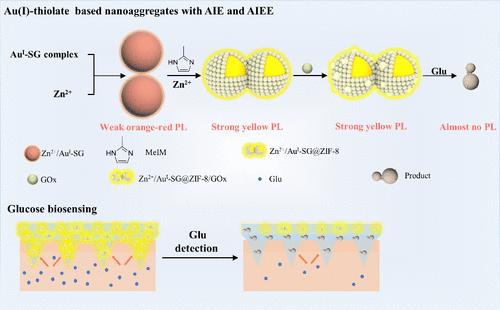
逐步点亮金(I) -硫酸盐配合物从AIE纳米聚集体到具有ZIF-8外壳的AIEE纳米探针用于葡萄糖生物传感
聚集诱导发射(AIE)或聚集诱导发射增强(AIEE)使金具有响应性荧光特性,有利于其在传感、成像和治疗方面的潜在应用。然而,制造同时具有AIE和AIEE效应的荧光团仍然是一个有趣的挑战。在此,我们提出了具有Zn2+诱导aiee和咪唑酸分子筛框架(ZIF-8)诱导aiee效应的高发光Au(I)噻唑酸纳米配合物生物传感器。通过配位辅助自组装策略,获得了非发光的单价金-谷胱甘肽配合物(AuI-SGs),合成了强发光的核壳Zn2+/AuI-SG@ZIF-8复合材料。通过将GOx固定在Zn2+/AuI-SG@ZIF-8表面,Zn2+/AuI-SG@ZIF-8/GOx生物传感器对葡萄糖表现出有效的响应性,显示出“关闭”检测模型。机理研究表明,Zn2+/AuI-SG@ZIF-8对葡萄糖传感的强大发光归因于葡萄糖氧化酶(GOx)催化氧化过程中产生的H+促进了探针的酸刺激降解。为了实现无创智能血糖检测,我们进一步开发了负载Zn2+/AuI-SG@ZIF-8/ gox的微针(MN)贴片荧光平台。基于mn贴片的传感平台在针上捕获和原位葡萄糖检测方面具有良好的性能。本研究展示了一种普遍可行的方案来构建用于葡萄糖检测的发光生物传感器,以及它们在基于mn的分析设备开发中的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


