Macrocyclic Peptide-Based Dual-Sensor Platform for Linkage-Specific Visualization of Ubiquitin Chain Assembling in Live Cells
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Intracellular monitoring of protein ubiquitination and differentiating polyubiquitin chain topology are crucial for understanding life processes and drug discovery, which is challenged by the high complexity of the ubiquitination process and a lack of molecular tools. Herein, a synthetic dual-sensor platform specific for K48-linked ubiquitin oligomers was tailored for in situ visualization of polyubiquitin chain assembling in live biosystems. This is achieved using macrocyclic peptides as recognition motifs and a tetraphenylethylene derivative as an activatable reporter. The efficient cell penetration, tight binding, and protection of polyubiquitin delivered a “freeze-and-image” approach, allowing fluorescent readout of polyubiquitin linkage type and chain elongation without perturbing the physiological environment. Motivated by these unique features, mapping of K48-ubiquitination dynamics during protein degradation was facilely achieved. Rapid, sensitive, and intracellular assessment of the mechanism of action and potency dependence for proteolysis-targeting chimeras (PROTACs) was demonstrated, presenting the sensors as promising molecular tools for PROTAC drug development.
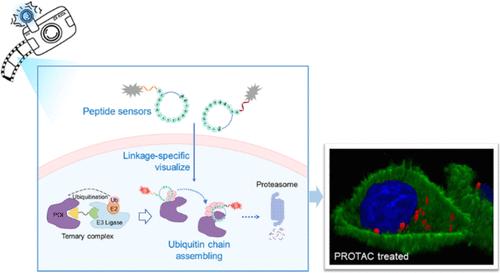
基于大环肽的双传感器平台,用于活细胞中泛素链组装的链接特异性可视化
细胞内监测蛋白质泛素化和区分多泛素链拓扑结构对于了解生命过程和药物发现至关重要,但泛素化过程的高度复杂性和分子工具的缺乏给这一工作带来了挑战。在这里,我们为 K48 链接的泛素寡聚体定制了一个合成的双传感器平台,用于现场生物系统中多泛素链组装的原位可视化。该平台使用大环肽作为识别图案,使用四苯基乙烯衍生物作为可激活的报告物。多泛素的高效细胞渗透、紧密结合和保护提供了一种 "冷冻-成像 "方法,允许在不干扰生理环境的情况下对多泛素连接类型和链延长进行荧光读数。在这些独特功能的推动下,蛋白质降解过程中的 K48 泛素化动态制图得以轻松实现。对蛋白水解靶向嵌合体(PROTACs)的作用机制和效力依赖性进行了快速、灵敏和细胞内评估,证明传感器是开发 PROTAC 药物的理想分子工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


