Aptamer-Driven Multifunctional Nanoplatform for Near-Infrared Fluorescence Imaging and Rapid In Situ Inactivation of Salmonella typhimurium
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
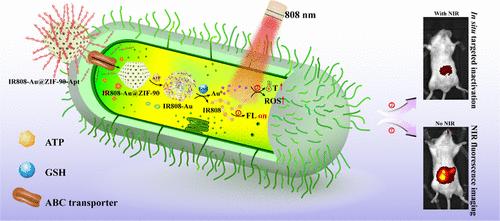
Salmonella typhimurium (S. typhimurium) is a prominent pathogen responsible for intestinal infections, primarily transmitted through contaminated food and water. This underscores the critical need for precise and biocompatible technologies enabling early detection and intervention of bacterial colonization in vivo. Herein, a multifunctional nanoplatform (IR808-Au@ZIF-90-Apt) was designed, utilizing an S. typhimurium-specific aptamer to initiate cascade responses triggered by intracellular ATP and GSH. The nanoplatform precisely targets S. typhimuriumvia aptamer recognition, promoting bacterial aggregation through nanoparticle sedimentation in an oscillatory system. Furthermore, the intelligent nanoplatform significantly enhances the sensitivity of S. typhimurium detection based on near-infrared (NIR) fluorescence signals, achieving a detection limit as low as 2 CFU mL–1. Additionally, in situ NIR irradiation was applied at the 30 min peak of fluorescence detection, enabling rapid and irreversible inactivation of S. typhimurium through the synergistic effects of photothermal and photodynamic effects. Importantly, in a mouse model of intestinal infection, the nanoplatform successfully detected early S. typhimurium colonization and achieved highly efficient in situ inactivation without adversely affecting the major organs. In conclusion, the nanoplatform achieved precise localized detection and in situ inactivation of S. typhimurium, offering valuable insights for disease surveillance and epidemiological studies, with promising implications for food safety and public health.
用于鼠伤寒沙门氏菌近红外荧光成像和快速原位灭活的色素驱动多功能纳米平台
鼠伤寒沙门氏菌(S. typhimurium)是引起肠道感染的主要病原体,主要通过受污染的食物和水传播。这强调了对精确和生物相容性技术的迫切需要,这些技术能够早期检测和干预细菌在体内的定植。本文设计了一个多功能纳米平台(IR808-Au@ZIF-90-Apt),利用鼠伤寒沙门氏菌特异性适配体启动细胞内ATP和GSH触发的级联反应。该纳米平台通过适配体识别精确靶向鼠伤寒沙门氏菌,在振荡系统中通过纳米粒子沉降促进细菌聚集。此外,智能纳米平台显著提高了基于近红外(NIR)荧光信号的鼠伤寒沙门氏菌检测的灵敏度,检测限低至2 CFU mL-1。此外,在荧光检测的30 min峰值处进行原位近红外照射,通过光热效应和光动力效应的协同作用,实现了鼠伤寒沙门氏菌的快速、不可逆灭活。重要的是,在小鼠肠道感染模型中,纳米平台成功检测了早期鼠伤寒沙门氏菌的定植,并在不影响主要器官的情况下实现了高效的原位失活。总之,该纳米平台实现了鼠伤寒沙门氏菌的精确定位检测和原位灭活,为疾病监测和流行病学研究提供了有价值的见解,对食品安全和公共卫生具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


