Integrative Quantitative Analysis of Platelet Proteome and Site-Specific Glycoproteome Reveals Diagnostic Potential of Platelet Glycoproteins for Liver Cancer
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
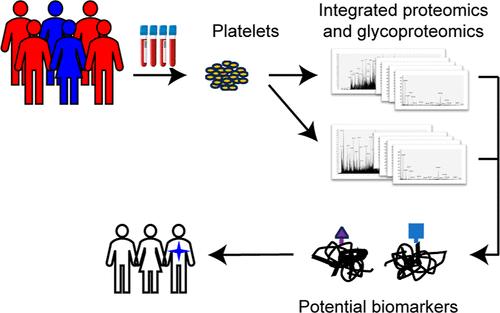
The role of peripheral blood platelets as indicators of cancer progression is increasingly recognized, and the significance of abnormal glycosylation in platelet function and related disorders is gaining attention. However, the potential of platelets as a source of protein site-specific glycosylation for cancer diagnosis remains underexplored. In this study, we proposed a general pipeline that integrates quantitative proteomics with site-specific glycoproteomics, allowing for an in-depth investigation of the platelet glycoproteome. With this pipeline, we generated a data set comprising 3,466 proteins with qualitative information, 3,199 proteins with quantitative information, 3,419 site-specific glycans with qualitative information and 3,377 site-specific glycans with quantitative information from peripheral blood platelets of hepatocellular carcinoma (HCC) patients, metastatic liver cancer (mLC) patients, and healthy controls. The integrated analysis revealed significant changes in platelet protein N-glycosylation in liver cancer patients. Further systems biology analysis and lectin pull-down-coupled ELISA assays in independent clinical samples confirmed two N-glycoproteins with specific glycan types, complement C3 (C3) with oligomannose modification and integrin β-3 (ITGB3) with sialylation, as potential biomarkers distinguishing liver cancer patients from healthy individuals, without differentiating between HCC and mLC patient group. These findings highlight the potential of platelet protein glycosylation as biomarkers.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


