iTASO: A Novel Photosensitive Probe for High-Throughput and Selective Submetabolomic Analysis via Flow Injection-Mass Spectrometry
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
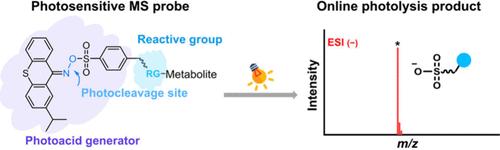
Flow injection mass spectrometry (FI-MS) is widely employed for high-throughput metabolome analysis, yet the absence of prior separation leads to significant matrix effects, thereby limiting the metabolome coverage. In this study, we introduce a novel photosensitive MS probe, iTASO-ONH2, integrated with FI-MS to establish a high-throughput strategy for submetabolome analyses. The iTASO probe features a conjugated-imino sulfonate moiety for efficient photolysis under 365 nm irradiation and a reactive group for selective metabolite labeling. The iTASO-ONH2 probe effectively and selectively labels carbonyl compounds, forming highly stable labeled products. Upon UV exposure, the labeled products rapidly release sulfonic acid-containing photolysis products, detectable with high sensitivity in ESI-negative mode and low matrix effect, offering femtomole-level detection sensitivity. The iTASO-ONH2-based FI-MS strategy was applied to fecal samples from chronic sleep-deprived and control mice, revealing 192 potential carbonyl compounds of which 37 exhibited significant alterations. Additionally, three other photosensitive probes─iTASO-NH2, iTASO-NHS, and iTASO-MAL─were synthesized to selectively label carboxyl, amino, and thiol metabolites, respectively, underscoring the versatility of the iTASO-based FI-MS strategy for submetabolomic analysis across diverse metabolite classes.
iTASO:一种新型光敏探针,用于高通量和选择性的流动注射-质谱亚代谢组学分析
流动注射质谱(FI-MS)被广泛用于高通量代谢组分析,但由于没有事先分离,导致明显的基质效应,从而限制了代谢组的覆盖范围。在这项研究中,我们引入了一种新型光敏质谱探针iTASO-ONH2,它与FI-MS相结合,建立了一种高通量的亚代谢组分析策略。iTASO探针具有在365 nm照射下进行有效光解的共轭亚胺磺酸基团和用于选择性代谢物标记的活性基团。iTASO-ONH2探针可以有效和选择性地标记羰基化合物,形成高度稳定的标记产物。在紫外线照射下,标记的产品快速释放含磺酸的光解产物,在esi阴性模式下具有高灵敏度和低基质效应,具有飞摩尔级的检测灵敏度。基于itaso - onh2的FI-MS策略应用于慢性睡眠剥夺小鼠和对照组的粪便样本,发现192个潜在的羰基化合物,其中37个表现出显著的变化。此外,还合成了另外三种光敏探针──iTASO-NH2、iTASO-NHS和iTASO-MAL──分别用于选择性标记羧基、氨基和硫醇代谢物,强调了基于itaso的FI-MS策略在不同代谢物类别的亚代谢组学分析中的多功能性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


