Amplification-Free Attomolar Detection of Short Nucleic Acids with Upconversion Luminescence: Eliminating Nonspecific Binding by Hybridization Complex Transfer
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
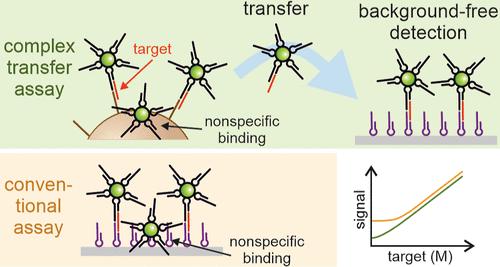
The anti-Stokes emission of photon upconversion nanoparticles (UCNPs) facilitates their use as labels for ultrasensitive detection in biological samples as infrared excitation does not induce autofluorescence at visible wavelengths. The detection of extremely low-abundance analytes, however, remains challenging as it is impossible to completely avoid nonspecific binding of label conjugates. To overcome this limitation, we developed a novel hybridization complex transfer technique using UCNP labels to detect short nucleic acids directly without target amplification. The assay involves capturing the target–label complexes on an initial solid phase, then using releasing oligonucleotides to specifically elute only the target–UCNP complexes and recapturing them on another solid phase. The nonspecifically adsorbed labels remain on the first solid phase, enabling background-free, ultrasensitive detection. When magnetic microparticles were used as the first solid phase in a sample volume of 120 μL, the assay achieved a limit of detection (LOD) of 310 aM, a 27-fold improvement over the reference assay without transfer. Moreover, the additional target-specific steps introduced in the complex transfer procedure improved the sequence specificity of the complex transfer assay compared with the reference assay. The suitability for clinical analysis was confirmed using spiked plasma samples, resulting in an LOD of 190 aM. By increasing the sample volume to 600 μL and using magnetic preconcentration, the LOD was improved to 46 aM. These results highlight the importance of background elimination in achieving ultralow LODs for the analysis of low-abundance biomarkers.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


