Operando Photoelectrochemical Surface-Enhanced Raman Spectroscopy: Interfacial Mechanistic Insights and Simultaneous Detection of Patulin
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Comprehending the biosensing mechanism of the biosensor interface is crucial for sensor development, yet accurately reflecting interfacial interactions within actual detection environments remains an unsolved challenge. An operando photoelectrochemical surface-enhanced Raman spectroscopy (PEC-SERS) biosensing platform was developed, capable of simultaneously capturing photocurrent and SERS signals, allowing operando characterization of the interfacial biosensing behavior. Porphyrin-based MOFs (Zr-MOF) served as bifunctional nanotags, providing a photocurrent and stable Raman signal output under 532 nm laser irradiation. Aptamer was used to bridge the Zr-MOF and the silver-encased gold nanodumbbells (AuNDs@AgNPs). The simultaneous in situ acquisition of target-induced PEC and SERS signal responses facilitated the correlation of electron transfer information from the photocurrent with the distance information from the SERS signal. It revealed the biosensing mechanism in which target-induced aptamer conformational bending drove the Zr-MOF to approach the electrode. However, the increase in charge transfer observed through conventional electrochemical methods contradicts the conclusions drawn from the operando PEC-SERS analysis. Comprehensive analysis indicated that redox probes introduced during the non-in-situ measurement process became adsorbed within the MOF pores, potentially affecting the judgment of the biosensing mechanism. In addition, the operando PEC-SERS biosensor simultaneously obtained two independent signals, providing self-verification to improve the accuracy and reliability of patulin detection. The linear ranges were 1 pg mL–1–10 ng mL–1 for the PEC method and 1 pg mL–1–100 ng mL–1 for the SERS method, respectively. This work provides a powerful tool for determining the interface characteristics of biosensors.
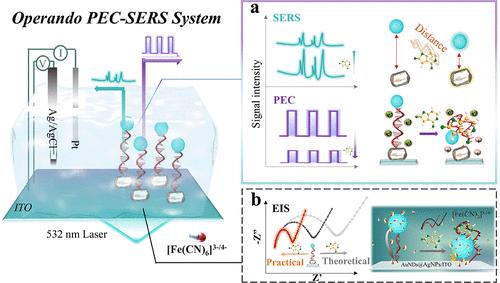
Operando光电化学表面增强拉曼光谱:界面机理的见解和展霉素的同时检测
理解生物传感器界面的生物传感机制对传感器的发展至关重要,但在实际检测环境中准确反映界面相互作用仍然是一个未解决的挑战。开发了一个operando光电化学表面增强拉曼光谱(PEC-SERS)生物传感平台,能够同时捕获光电流和SERS信号,允许operando表征界面生物传感行为。基于卟啉的mof (Zr-MOF)作为双功能纳米标签,在532 nm激光照射下提供光电流和稳定的拉曼信号输出。适配体被用来桥接Zr-MOF和银包裹的金纳米哑铃(AuNDs@AgNPs)。同时原位获取靶诱导的PEC和SERS信号响应,促进了来自光电流的电子转移信息与来自SERS信号的距离信息的相关性。揭示了靶诱导的适体构象弯曲驱动Zr-MOF向电极靠近的生物传感机制。然而,通过常规电化学方法观察到的电荷转移增加与operando PEC-SERS分析得出的结论相矛盾。综合分析表明,在非原位测量过程中引入的氧化还原探针被吸附在MOF孔内,可能会影响生物传感机制的判断。此外,operando PEC-SERS生物传感器同时获得两个独立的信号,提供自我验证,提高了展霉素检测的准确性和可靠性。PEC法和SERS法的线性范围分别为1 pg mL-1 ~ 10 ng mL-1和1 pg mL-1 ~ 100 ng mL-1。这项工作为确定生物传感器的界面特性提供了有力的工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
文献相关原料
公司名称
产品信息
阿拉丁
Zirconyl chloride octahydrate
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


