Comprehensive Approach for Sequential MALDI-MSI Analysis of Lipids, N-Glycans, and Peptides in Fresh-Frozen Rodent Brain Tissues
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
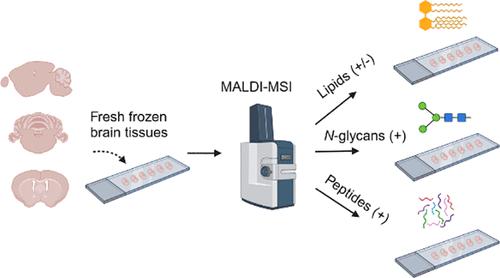
Multiomics analysis of single tissue sections using matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI) provides comprehensive molecular insights. However, optimizing tissue sample preparation for MALDI-MSI to achieve high sensitivity and reproducibility for various biomolecules, such as lipids, N-glycans, and tryptic peptides, presents a significant challenge. This study introduces a robust and reproducible protocol for the comprehensive sequential analysis of the latter molecules using MALDI-MSI in fresh-frozen rodent brain tissue samples. The optimization process involved testing multiple organic solvents, which identified serial washing in ice-cold methanol, followed by chloroform as optimal for N-glycan analysis. Integrating this optimized protocol into MALDI-MSI workflows enabled comprehensive sequential analysis of lipids (in dual polarity mode), N-glycans, and tryptic peptides within the same tissue sections, enhancing both the efficiency and reliability. Validation across diverse rodent brain tissue samples confirmed the protocol’s robustness and versatility. The optimized methodology was subsequently applied to a transgenic Alzheimer’s disease (AD) mouse model (tgArcSwe) as a proof of concept. In the AD model, significant molecular alterations were observed in various sphingolipid and glycerophospholipid species, as well as in biantennary and GlcNAc-bisecting N-glycans, particularly in the cerebral cortex. These region-specific alterations are potentially associated with amyloid-beta (Aβ) plaque accumulation, which may contribute to cognitive and memory impairments. The proposed standardized methodology represents a significant advancement in neurobiological research, providing valuable insights into disease mechanisms and laying the foundation for potential preclinical applications. It could aid the development of diagnostic biomarkers and targeted therapies for AD and other neurodegenerative diseases, such as Parkinson’s disease.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


