Advanced Dual-Mode Microfluidic Sensing Platform Based on Amphiphilic Polymer-Capped Perovskite Nanozymes Induced Photoelectrochemical Signal Amplification and Fluorescence Emission
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
A novel dual-mode microfluidic sensing platform integrating photoelectrochemical (PEC) and fluorescence (FL) sensors was developed for the sensitive monitoring of heart fatty acid binding protein (h-FABP). First, BiVO4/AgInS2 (BVAIS) composites with excellent photoelectric activity were synthesized as sensing matrices. The BVAIS heterojunction with a well-matched internal energy level structure provided a stable photocurrent. Second, an innovative signal amplification strategy based on octylamine-modified poly(acrylic acid) (OPA)-capped CsPbBr3 (OPCB) nanocrystals (NCs) with excellent catalytic activity and fluorescence property was proposed. On the OPCB nanozyme possessing ascorbate oxidase-like catalytic activity could catalyze the oxidation of ascorbic acid, which achieved quenching of the photocurrent signals by competitively consuming the electron donor. On the other hand, the OPCB NCs that overcame the water stability defect processed good luminescence performance and were able to produce obvious FL signals. Mutually verified dual-response signals effectively enhance the precision of test outcomes and avoid false-positive or false-negative results. Finally, the constructed microfluidic sensing platform realized sensitive detection of h-FABP in the linear range of 0.0001–150 ng/mL (PEC mode) and 0.001–150 ng/mL (FL mode), with detection limits of 36 fg/mL and 0.32 pg/mL, respectively. The present work provided a new perspective for designing an efficient dual-mode sensing strategy to achieve sensitive detection of disease markers.
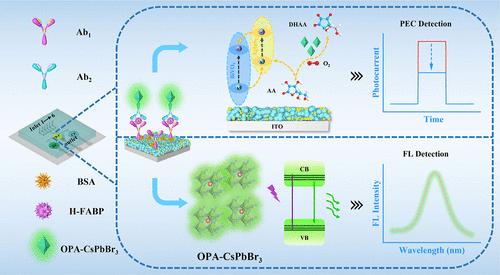
基于两亲性聚合物覆盖的钙钛矿纳米酶诱导光电信号放大和荧光发射的先进双模微流控传感平台
研制了一种集成光电化学(PEC)和荧光(FL)传感器的新型双模微流控传感平台,用于心脏脂肪酸结合蛋白(h-FABP)的灵敏监测。首先,合成具有优异光电活性的BiVO4/AgInS2 (BVAIS)复合材料作为传感基质。具有良好匹配内部能级结构的BVAIS异质结提供了稳定的光电流。其次,提出了一种基于辛胺修饰聚丙烯酸(OPA)包封CsPbBr3 (OPCB)纳米晶体(NCs)的新型信号放大策略,该纳米晶体具有优异的催化活性和荧光性能。具有抗坏血酸氧化酶样催化活性的OPCB纳米酶可以催化抗坏血酸的氧化,通过竞争性消耗电子供体实现光电流信号的猝灭。另一方面,克服了水稳定性缺陷的OPCB nc处理了良好的发光性能,能够产生明显的FL信号。相互验证的双响应信号有效地提高了检测结果的精度,避免了假阳性或假阴性结果。最后,构建的微流控传感平台实现了h-FABP在0.0001 ~ 150 ng/mL (PEC模式)和0.001 ~ 150 ng/mL (FL模式)线性范围内的灵敏检测,检出限分别为36 fg/mL和0.32 pg/mL。本工作为设计一种高效的双模传感策略来实现疾病标志物的灵敏检测提供了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


