Development of Citric-Acid-Modified Cellulose Monolith for Enriching Glycopeptides
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
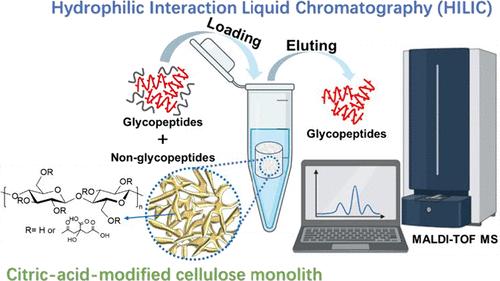
Prior to mass spectrometry (MS) analysis, pretreatment of low-abundance glycopeptides is vital for identifying protein glycosylation. In this study, we fabricated an environmentally friendly citric-acid-modified cellulose monolith (CCM) characterized by a coral-like porous structure and high-density hydrophilic groups using a thermally induced phase separation (TIPS) method. The CCM production leverages biomass resources, specifically cellulose and citric acid, utilizing TIPS to synthesize continuous porous materials through a straightforward heating and cooling process of polymer solutions. We demonstrated the efficacy of CCM as a hydrophilic interaction liquid chromatography (HILIC) medium for the efficient enrichment of glycopeptides. It exhibited remarkable selectivity in enriching glycopeptides from trypsin-digested immunoglobulin G (IgG), serving as a model protein, even in the presence of a significant amount of non-glycopeptide contaminants from bovine serum albumin (BSA) at a ratio of BSA/IgG of 1000/1. Additionally, CCM showed a low detection limit (0.25 fmol μL–1) and commendable reusability in glycopeptide enrichment, successfully enriching 35 glycopeptides from IgG. Additionally, 641 unique N-glycosylation sites of 698 unique glycopeptides from 393 glycosylated proteins were identified from the triplicate analysis of 900 μg of human hepatocellular carcinoma tissue. Therefore, CCM holds significant promise as an eco-friendly stationary phase for hydrophilic interaction liquid chromatography aimed at glycopeptide enrichment.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


