Specific Protein Quantification by Radioimmuno-Dot-Blot Assay for Complex Mixture Samples Utilizing Strep-Tag and Tritium-Labeled Strep-Tactin
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
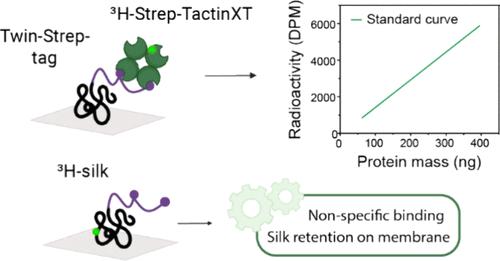
Accurately quantifying specific proteins from complex mixtures like cell lysates, for example, during in vivo studies, is difficult, especially for aggregation-prone proteins. Herein, we describe the development of a specific protein quantification method that combines a solid-state dot blot approach with radiolabel detection via liquid scintillation counting. The specific detection with high sensitivity is achieved by using the Twin-Strep protein affinity tag and tritium-labeled 3HStrep-TactinXT probe. While the assay was developed with the recombinant silk protein CBM-AQ12-CBM as a target, the method can be adapted to other recombinant proteins. Variations of the protein tag and Strep-Tactin probe were tested, and it was found that only the combination of Strep-TactinXT and Twin-Strep-tag performed adequately: with this combination, a precision of 95% and an accuracy of 86% were achieved with a linear region from 19 to 400 ng and a limit of quantification at 0.4 pmol. To achieve this, critical optimization steps were preventing nonspecific adsorption and promoting surface adhesion of the target protein to the solid nitrocellulose membrane. The often-overlooked challenges of sample preparation and protein immobilization in quantification assays are discussed and insights into overcoming such issues are provided.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


