Thermo-reversible gelation and enhanced umami perception of myofibrillar proteins induced by protein-glutaminase-mediated deamidation
IF 8.5
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
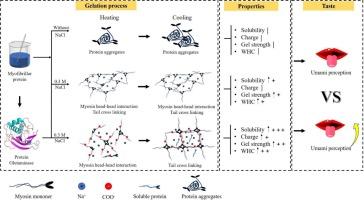
In this study, the absolute electrostatic charge of myofibrillar protein (MP) was substantially increased by protein-glutaminase (PG) treatment, which was a critical step for achieving the dissociation and solubility of MP under low salt condition. The PG-treated MP exhibited the capacity to form thermo-reversible gels that could be melted through heating and subsequently reformed into a stable gel structure upon refrigeration. The results of SDS-PAGE further revealed that the levels of soluble monomeric myosin and actin in the supernatant of deamidated MP (DMP) gels were markedly elevated, and confirmed the increased formation of intermolecular disulfide bond between myosin and actin. Additionally, moderate deamidation was beneficial for the improvements of MP gel properties, especially in terms of water-holding capacity and springiness. Electronic tongue and correlation analysis indicated that the umami perception of DMP was significantly enhanced because of the conversion of glutamine (Gln) to glutamate (Glu) residues that induced by PG deamidation.

由蛋白谷氨酰胺酶介导的脱酰胺诱导的肌纤维蛋白热可逆凝胶化和增强鲜味感知
在本研究中,蛋白谷氨酰胺酶(PG)处理显著增加了肌原纤维蛋白(MP)的绝对静电荷,这是实现MP在低盐条件下解离和溶解的关键步骤。经过pg处理的MP表现出形成热可逆凝胶的能力,这种凝胶可以通过加热融化,随后在冷藏后转化为稳定的凝胶结构。SDS-PAGE进一步显示脱酰胺MP (DMP)凝胶上清液中可溶性单体肌球蛋白和肌动蛋白水平明显升高,证实肌球蛋白和肌动蛋白之间的分子间二硫键形成增加。此外,适度的脱酰胺处理有利于MP凝胶性能的改善,特别是在持水能力和弹性方面。电子舌和相关分析表明,由于PG脱酰胺诱导谷氨酰胺(Gln)转化为谷氨酸(Glu)残基,DMP的鲜味感知显著增强。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


