Design, Synthesis, and Biological Activity Evaluation of 5-Aryl-cyclopenta[c]pyridine Derivatives
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Taking the natural product cerbinal as the lead compound, 30 novel 5-aryl-cyclopenta[c]pyridine derivatives were designed and synthesized based on the previous bioactivity studies of the cyclopenta[c]pyridines. The modification of the position-5 of compound 2 was achieved by amination, bromination, and cross coupling using cerbinal as the raw material. The results of the bioactivity tests demonstrated that partial compounds exhibited superior activity against plant viruses compared to compound 2. Compounds 4g and 4k showed higher anti-TMV activity levels than commercial varieties of ribavirin at concentrations of 500 and 100 μg/mL. In particular, compound 4k, which contained a m-methoxyphenyl substitution, displayed the most potent anti-TMV activity in vivo (inactivation effect 51.1 ± 1.9%, curative effect 50.7 ± 3.6%, protection effect 53.8 ± 2.8% at 500 μg/mL) The toxicological experiments also revealed that compound 4k exhibited low toxicity to zebrafish. Additionally, molecular docking results indicated that access to the benzene ring enhanced the binding affinity of these derivatives for TMV receptor proteins. Furthermore, studies on the insecticidal and fungicidal activities of these derivatives showed that most of the compounds exhibited good larvicidal efficacy against Plutella xylostella and broad-spectrum fungicidal activities. Notably, compound 4i (3,4,5-trifluorophenyl) displayed an outstanding inhibition ratio of 91.9% against Sclerotinia sclerotiorum, 75% against Botrytis cinerea, and 62.5% against Phytophthora infestans at a concentration of 50 μg/mL. These results suggest that 5-aryl-cyclopenta[c]pyridine derivatives could serve as promising candidate agents for antiviral, insecticidal, and fungicidal applications in agricultural production.
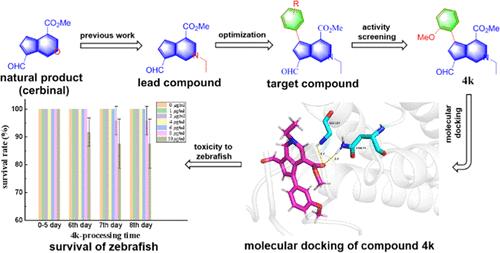
5-芳基环戊[c]吡啶衍生物的设计、合成及生物活性评价
以天然产物cerbinal为先导化合物,在前人对环五[c]吡啶生物活性研究的基础上,设计合成了30个新的5-芳基环五[c]吡啶衍生物。以甲醇为原料,通过胺化、溴化和交叉偶联对化合物2的5位进行了修饰。生物活性试验结果表明,部分化合物的抗植物病毒活性优于化合物2。在500和100 μg/mL浓度下,化合物4g和4k的抗tmv活性高于市售品种。其中,含间甲氧基苯基取代的化合物4k在500 μg/mL时抗tmv活性最强(灭活效果51.1±1.9%,疗效50.7±3.6%,保护效果53.8±2.8%),毒理学实验也表明,化合物4k对斑马鱼具有较低的毒性。此外,分子对接结果表明,进入苯环增强了这些衍生物与TMV受体蛋白的结合亲和力。此外,对这些衍生物的杀虫和杀真菌活性的研究表明,大多数化合物对小菜蛾具有良好的杀幼虫效果和广谱的杀真菌活性。值得注意的是,化合物4i(3,4,5-三氟苯基)在浓度为50 μg/mL时,对菌核菌(Sclerotinia sclerotiorum)、灰霉病菌(Botrytis cinerea)和疫霉(Phytophthora infestans)的抑制率分别为91.9%、75%和62.5%。这些结果表明,5-芳基环戊烷[c]吡啶衍生物在农业生产中具有抗病毒、杀虫和杀真菌的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


