Targeted Adherence and Enhanced Degradation of Feather Keratins by a Novel Prepeptidase C-Terminal Domain-Fused Keratinase
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
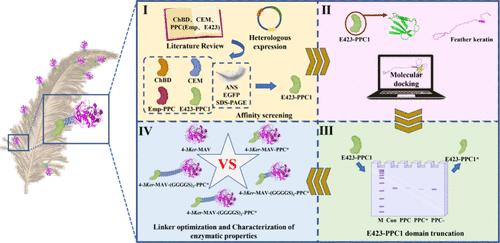
Keratinases are valuable enzymes for converting feather keratin waste into bioactive products but often suffer from poor substrate specificity and low catalytic efficiency. This study reported the creating of a novel keratinase with targeted adherence and specific degradation on feather keratins by fusing prepeptidase C-Terminal (PPC) domain. A PPC domain of metalloprotease E423 specifically adsorbed feather keratins by hydrogen bonds and hydrophobic interactions in a time- and temperature-dependent manner. Stepwise N-/C-terminal truncations disclosed the essential core sequence composed of 21 amino acid residues determining the keratin-targeted adherence. Fusion of the core fragment with a flexible linker (GGGGS)1 achieved the optimal secretion, and improved the catalytic efficiency of a representative keratinase 4-3Ker-MAV by 0.97-fold. Moreover, the feather degradation rate increased from 65 to 82%, representing the highest reported performance for a keratinase. This PPC-fusion strategy opens new horizons in enzyme engineering, promising not only to revolutionize keratin waste valorization but also to inspire the design of substrate-specific biocatalysts across diverse industrial applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


