The brain’s action-mode network
IF 26.7
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
The brain is always intrinsically active, using energy at high rates while cycling through global functional modes. Awake brain modes are tied to corresponding behavioural states. During goal-directed behaviour, the brain enters an action-mode of function. In the action-mode, arousal is heightened, attention is focused externally and action plans are created, converted to goal-directed movements and continuously updated on the basis of relevant feedback, such as pain. Here, we synthesize classical and recent human and animal evidence that the action-mode of the brain is created and maintained by an action-mode network (AMN), which we had previously identified and named the cingulo-opercular network on the basis of its anatomy. We discuss how rather than continuing to name this network anatomically, annotating it functionally as controlling the action-mode of the brain increases its distinctiveness from spatially adjacent networks and accounts for the large variety of the associated functions of an AMN, such as increasing arousal, processing of instructional cues, task general initiation transients, sustained goal maintenance, action planning, sympathetic drive for controlling physiology and internal organs (connectivity to adrenal medulla), and action-relevant bottom–up signals such as physical pain, errors and viscerosensation. In the functional mode continuum of the awake brain, the AMN-generated action-mode sits opposite the default-mode for self-referential, emotional and memory processing, with the default-mode network and AMN counterbalancing each other as yin and yang. The brain enters an action-mode of function during goal-directed behaviour. In this Perspective, Dosenbach, Raiche and Gordon describe “action-mode” as an informative functional label that reduces anatomical network naming confusion, then characterize how the reannotated action-mode network supporting it counterbalances the default mode network.

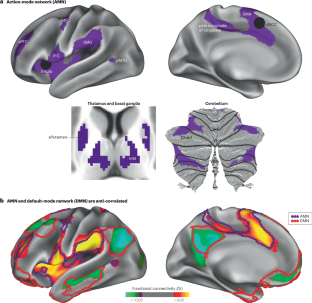
大脑的行动模式网络
大脑本质上总是活跃的,在全球功能模式循环的同时以高速率消耗能量。清醒的大脑模式与相应的行为状态相关联。在目标导向的行为中,大脑进入一种行动模式的功能。在行动模式中,唤醒被提高,注意力被集中在外部,行动计划被创建,转化为目标导向的运动,并根据相关反馈(如疼痛)不断更新。在这里,我们综合了经典的和最近的人类和动物的证据,证明大脑的动作模式是由一个动作模式网络(AMN)创建和维持的,我们之前在其解剖学的基础上确定并命名为扣谷-眼窝网络。我们讨论了如何不再继续从解剖学上命名这个网络,而是在功能上将其注释为控制大脑的行动模式,增加其与空间相邻网络的独特性,并解释了AMN的各种相关功能,如增加唤醒,处理教学线索,任务一般启动瞬态,持续目标维持,行动计划,控制生理和内部器官(连接到肾上腺髓质)的交感驱动,以及与动作相关的自下而上信号,如身体疼痛、错误和内脏感觉。在清醒大脑的功能模式连续体中,AMN产生的动作模式与自我参照、情绪和记忆处理的默认模式相对立,默认模式网络和AMN作为阴阳相互平衡。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Neuroscience
NEUROSCIENCES-
自引率
0.60%
发文量
104
期刊介绍:
Nature Reviews Neuroscience is a multidisciplinary journal that covers various fields within neuroscience, aiming to offer a comprehensive understanding of the structure and function of the central nervous system. Advances in molecular, developmental, and cognitive neuroscience, facilitated by powerful experimental techniques and theoretical approaches, have made enduring neurobiological questions more accessible. Nature Reviews Neuroscience serves as a reliable and accessible resource, addressing the breadth and depth of modern neuroscience. It acts as an authoritative and engaging reference for scientists interested in all aspects of neuroscience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


