Novel Nanoliposomes Synergistically Modulated by Sitogluside and Dioscin: Stability, Bioavailability, and Capacity To Alleviate Hyperuricaemia
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
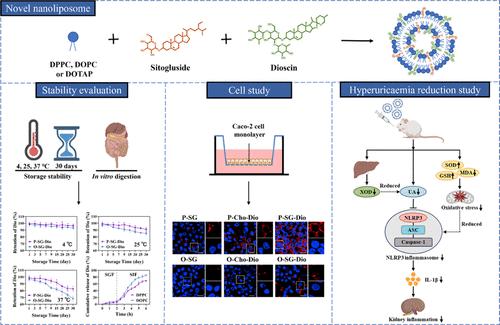
Cholesterol (Cho) is commonly used to stabilize nanoliposomes; however, there is controversy on the relationship between Cho and health. In this study, we developed a novel multifunctional nanoliposome utilizing structurally similar sitogluside (SG) and dioscin (Dio) instead of Cho to anchor the phospholipid bilayer and synergistically modulate the membrane properties of the nanoliposome (DPPC or DOPC). The storage and gastrointestinal tract stability experiment demonstrated that the changes of physical and chemical properties, including the significantly reduced size and Dio retention rate of nanoliposomes synergistically modulated by SG and Dio compared to those of SG alone, regulated nanoliposomes. Moreover, the stabilization effect of DPPC nanoliposomes under the synergistic modulation of SG and Dio was superior to that of DOPC nanoliposomes. Similarly, in cell internalization and permeability studies, DPPC-sitogluside-dioscin (P-SG-Dio), which was synergistically modulated by SG and Dio, had the highest cellular uptake and transepithelial transport. In addition, compared with DPPC-cholesterol-dioscin (P-Cho-Dio) and free Dio, intragastric administration of P-SG-Dio for 14 days could effectively inhibit the activation of the NLRP3 inflammatory pathway in the kidney of hyperuricemic mice, exhibiting the best antihyperuricemic and anti-inflammatory effects. Fourier transform infrared and Raman spectroscopy results indicated that the glucose residues of SG and Dio synergistically modulate the membrane properties of nanoliposomes by forming hydrogen bonds between them and the polar heads of phospholipids. The absence of unsaturated bonds in DPPC led to the best effect of synergistic modulation, resulting in the superior membrane properties, stability, and bioavailability of P-SG-Dio. The finding offers valuable insight into the design and modification of nanoliposomes for the effective delivery of bioactive compounds.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


