Solar-Light-Mediated Phototransformation of Herbicide Tribenuron-Methyl Initiated by Its Coexisting Nitrate Ion in Sunlit Agricultural Drainages
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Understanding the environmental fate of chemical herbicides is crucial to sustainable agriculture. Due to their joint-use with nitrogen fertilizers, their residues often coexist with NO3– in agricultural drainages. In this study, tribenuron-methyl was used as a model to evaluate the role of NO3– in the phototransformation of chemical herbicides, which was characterized by a two-stage process. Initially, a slow hydrolysis occurs (kobs = 2.573 × 10–4 min–1), producing two hydrolysis products: methyl-2-(aminosulfonyl)-benzoate (MSB) and 2-methyl-4-methylamino-6-methoxy-1, 3, 5-triazine (MMT), which can be significantly accelerated by solar irradiation (kobs = 2.152 × 10–2 min–1). Subsequently, MSB undergoes a rapid NO3–-initiated photodegradation process (kobs = 2.251 × 10–2 min–1). MMT was identified as the refractory unit and undergoes a slow NO3–-initiated photodegradation process (kobs = 4.494 × 10–4 min–1). The underlying mechanisms were elucidated through electron paramagnetic resonance spectroscopy and reactive species quenching experiments. This study fills a knowledge gap on the interaction between NO3– and chemical herbicides, highlighting the pivotal role of NO3– in the phototransformation of chemical herbicides.
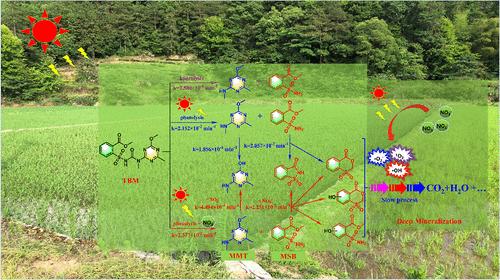
由共存的硝酸盐离子引发除草剂甲基三苯脲在日光农业排水中的光转化
了解化学除草剂的环境命运对可持续农业至关重要。由于它们与氮肥共同使用,在农业排水中,它们的残留物经常与NO3 -共存。本研究以甲基三苯脲为模型,考察了NO3 -在化学除草剂光转化过程中的作用,该过程分为两个阶段。最初,水解发生缓慢(kobs = 2.573 × 10-4 min-1),产生两种水解产物:甲基-2-(氨基磺酰基)苯甲酸酯(MSB)和2-甲基-4-甲氨基-6-甲氧基- 1,3,5 -三嗪(MMT),太阳照射可显著加速水解(kobs = 2.152 × 10-2 min-1)。随后,MSB经历了NO3引发的快速光降解过程(kobs = 2.251 × 10-2 min-1)。MMT被确定为难降解单元,经历了一个缓慢的NO3引发的光降解过程(kobs = 4.494 × 10-4 min-1)。通过电子顺磁共振波谱和活性物质猝灭实验对其机理进行了分析。本研究填补了NO3 -与化学除草剂相互作用的知识空白,突出了NO3 -在化学除草剂光转化中的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.
文献相关原料
公司名称
产品信息
阿拉丁
sucrose (SUC)
阿拉丁
bovine serum albumin (BSA)
阿拉丁
humic acid (HA)
阿拉丁
superoxide dismutase (SOD)
阿拉丁
1, 4-diazabicyclo[2.2.2]octane (DABCO)
阿拉丁
copper cyanide (CuCN)
阿拉丁
9, 10-anthracenediyl-bis (methylene) dimalonic acid (ABDA)
阿拉丁
terephthalic acid (TA)
阿拉丁
sodium nitrate (NaNO3)
阿拉丁
2-methyl-4-methylamino-6-methoxy-1, 3, 5-triazine (MMT)
阿拉丁
methyl-2-(aminosulfonyl)-benzoate (MSB)
阿拉丁
Tribenuron-methyl (TBM)
阿拉丁
sucrose (SUC)
阿拉丁
bovine serum albumin (BSA)
阿拉丁
humic acid (HA)
阿拉丁
superoxide dismutase (SOD)
阿拉丁
1, 4-diazabicyclo[2.2.2]octane (DABCO)
阿拉丁
copper cyanide (CuCN)
阿拉丁
9, 10-anthracenediyl-bis (methylene) dimalonic acid (ABDA)
阿拉丁
terephthalic acid (TA)
阿拉丁
sodium nitrate (NaNO3)
阿拉丁
2-methyl-4-methylamino-6-methoxy-1, 3, 5-triazine (MMT)
阿拉丁
methyl-2-(aminosulfonyl)-benzoate (MSB)
阿拉丁
Tribenuron-methyl (TBM)

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


