CRISPR/Cas12a-Powered Electrochemical Platform for Dual-miRNA Detection via an AND Logic Circuit
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
The CRISPR/Cas technology shows great potential in molecular detection and diagnostics. However, it is still challenging to detect multiple targets simultaneously using the CRISPR–Cas system. Herein, we ingeniously leverage the synergistic effect of two short single-stranded DNA activators to construct a CRISPR/Cas12a-driven electrochemical sensing platform based on an AND logic circuit (“AND” LC-CRISPR) for the simultaneous detection of dual miRNAs. Specifically, the exponential amplification reaction products triggered by the dual-specific miRNAs are designed as binary inputs to bind with Cas12a/crRNA, forming an AND logic circuit and activating the trans-cleavage ability of the CRISPR–Cas12a system. Subsequently, the hairpin probe biogate on the surface of the functionalized electrochemical signal probe (MB@HP-Fe-MOF) is cleaved by activated Cas12a, leading to the release of the encapsulated electroactive signal molecule methylene blue, thereby generating a strong electrochemical signal. As a result, this “AND” LC-CRISPR sensing platform, requiring only a single crRNA assembled with Cas12a, achieves simultaneous detection of miRNA-155 and miRNA-21 at concentrations as low as 3.2 fM. Moreover, the platform allows easy adjustment of the AND logic circuit inputs according to different detection targets, allowing it to be easily expanded for the analysis and diagnosis of other multibiomarkers. This approach demonstrates promising potential for future applications in intelligent diagnostic medicine.
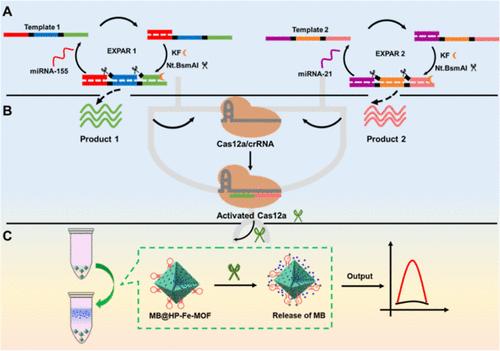
基于AND逻辑电路的CRISPR/ cas12a驱动的双mirna检测电化学平台
CRISPR/Cas技术在分子检测和诊断方面显示出巨大的潜力。然而,利用CRISPR-Cas系统同时检测多个靶标仍然是一个挑战。在此,我们巧妙地利用两个短单链DNA激活子的协同效应,构建了基于AND逻辑电路(“AND”LC-CRISPR)的CRISPR/ cas12a驱动的电化学传感平台,用于同时检测双miRNAs。具体而言,双特异性mirna触发的指数扩增反应产物被设计为与Cas12a/crRNA结合的二元输入,形成AND逻辑电路,激活CRISPR-Cas12a系统的反式切割能力。随后,功能化的电化学信号探针(MB@HP-Fe-MOF)表面的发夹探针生物门被活化的Cas12a劈裂,导致被封装的电活性信号分子亚甲基蓝释放出来,从而产生强烈的电化学信号。因此,这种“AND”LC-CRISPR传感平台只需要一个与Cas12a组装的crRNA,就可以在低至3.2 fM的浓度下同时检测miRNA-155和miRNA-21。此外,该平台允许根据不同的检测目标轻松调整AND逻辑电路输入,使其易于扩展以用于其他多生物标志物的分析和诊断。这种方法在智能诊断医学的未来应用中显示出很大的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


