Structural Feature of Salty/Saltiness-Enhancing Peptides Derived from Coprinus comatus and Their Stability during Subsequent Thermal Treatment and Maillard Reaction
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Through a quantitative analysis of saltiness perception, favorable enzymatic hydrolysis parameters were confirmed for the preparation of saltiness-enhancing peptide mixtures from Coprinus comatus. The enzymatic hydrolysate was fractionated into four fractions (F1–F4) by gel chromatography, with F3 exhibiting the strongest saltiness-enhancing effect (22% increase). LC-MS/MS analysis of F3 identified 36 peptides, and their secondary structures and interactions with the TMC4 receptor were examined through circular dichroism spectroscopy and molecular docking. Molecular docking analysis revealed Asn588, Ser165, Asp5, and Arg168 as key amino acid residues, with the peptide GDNVGF showing the lowest binding energy. Synthetic GDNVGF (0.01%) in 70 mmol/L NaCl enhanced saltiness by 17%. When 0.7% GDNVGF was added to the aqueous solution, its saltiness was equivalent to that of 36.89 mmol/L NaCl, which suggested that GDNVGF functions both as a saltiness-enhancing peptide and a salty peptide. The taste changes of peptides during thermal reactions were further investigated. The thermal stability of Coprinus comatus peptides was good, but their saltiness-enhancing effect slightly reduced due to thermal degradation. The Maillard reaction further diminished this effect, though the umami level remained satisfactory, offering new insights into using Coprinus comatus peptides as low-sodium salt substitutes.
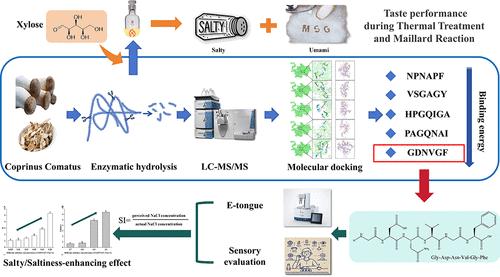
鸡尾螺含盐/增咸肽的结构特征及其在后续热处理和美拉德反应中的稳定性
通过对其咸味感知的定量分析,确定了适宜的酶解参数,以制备鸡毛茸增咸肽混合物。酶解产物经凝胶层析分为4个馏分(F1-F4),其中F3的增盐效果最强(增加22%)。LC-MS/MS分析鉴定了F3的36个肽段,并通过圆二色光谱和分子对接研究了它们的二级结构和与TMC4受体的相互作用。分子对接分析显示,Asn588、Ser165、Asp5和Arg168是关键氨基酸残基,其中肽GDNVGF的结合能最低。合成GDNVGF(0.01%)在70 mmol/L NaCl中使咸味提高17%。当0.7%的GDNVGF加入到水溶液中时,其咸度相当于36.89 mmol/L NaCl,说明GDNVGF既有增咸肽的功能,也有增咸肽的功能。进一步研究了热反应过程中多肽的口感变化。鸡毛茸肽的热稳定性较好,但其增盐作用因热降解而略有降低。美拉德反应进一步削弱了这种影响,但鲜味水平仍然令人满意,为使用鸡腿肽作为低钠盐替代品提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


