LRET-Based Simultaneous Detection of Dual miRNAs via Multitrap Optical Tweezers Assisted Suspension Array Tagged by Two Different Luminescent Quenchable UCNPs Combining CRISPR/Cas12a Amplification
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
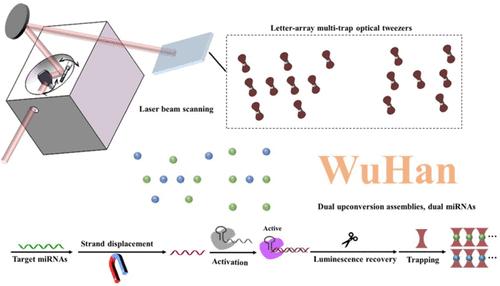
Nowadays, optical tweezers play a vital role not only in optical manipulation but also in bioassay. As principal optical trapping objects, microbeads can combine optical tweezers with suspension array technology, with amply focused laser beams and adequately concentrated tags contributing to highly sensitive detection. In view of the inefficiency of conventional single-trap optical tweezers, multitrap systems are developed. Here, green- and blue-emitting core–shell–shell upconversion nanoparticles (UCNPs) are adopted to encode microbeads and determine dual miRNAs, with the internal shells leading the luminescence process to facilitate quenching through luminescence resonance energy transfer (LRET). Utilizing the trans cleavage of CRISPR/Cas12a, quenched luminescence signals are recovered and amplified, causing further enhanced detection sensitivity. Ultimately, limits of detection (LOD) of 17 and 22 aM are obtained with excellent specificities verified. Furthermore, dual miRNAs from MCF-7, A549, and MCF-10A cells are extracted and detected, with results consistent with those obtained by PCR. Notably, miR-155 in MCF-7 and A549 cells is detectable at the single-cell level. Thus, the differences in the measured miRNA levels between MCF-7 and MCF-10A cells imply the potential of this method to discriminate breast cancer cells from epithelial cells despite the difficulty in distinguishing different cancer cells due to similar miRNA levels.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


