Near-Infrared Light-Activated DNA Nanodevice for Spatiotemporal In Vivo Fluorescence Imaging of Messenger RNA
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
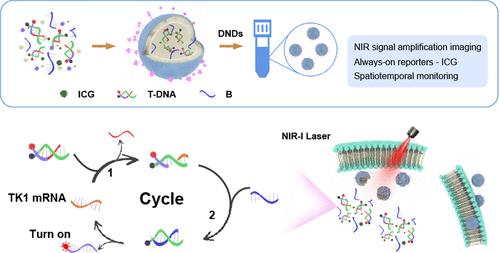
Real-time visualization of messenger RNA (mRNA) is essential for tumor classification, grading, and staging. However, the low signal-to-background ratios and nonspatiotemporal specific signal amplification restricted the in vivo imaging of mRNA. In this study, a near-infrared (NIR) light-activated DNA nanodevice (DND) was developed for spatiotemporal in vivo fluorescence imaging of mRNA. The DND was fabricated by encapsulating indocyanine green (ICG) and DNA fluorescent probes within thermosensitive liposomes and subsequently functionalizing the liposomes with aptamers. The ICG offers the “always-on” fluorescence signal, offering a feasible strategy for monitoring DND distribution. The fluorescence signal of DNA probes remains inactive (“off” state) during the delivery process. Upon targeted delivery of the DNDs to tumor cells via aptamer recognition, the thermosensitive liposomes could be dissociated by the photothermal effect induced by ICG under near-infrared irradiation, thereby facilitating the release of DNA probes. The DNA probes were activated (“turn on”) by tumor-specific thymidine kinase 1 (TK1) mRNA through toehold-mediated strand displacement cascades, enabling the signal-amplified fluorescence imaging of mRNA. This study reveals the distinctive light-activated merit and remarkable fluorescence imaging of DNDs, highlighting their great potential to promote progress in spatiotemporal resolution imaging of other disease-relevant RNAs in vivo.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


