Talarergosteroids A–C: Three Unusual Steroid-Polyketone Conjugates with Antifungal Activity from a Kandelia Obovata Derived Fungus Talaromyces sp
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Three previously undescribed steroid–polyketone conjugates, talarergosteroids A–C (1–3), together with talarergosteroid D (4), which was first identified from a natural source, were isolated from a Kandelia Obovata derived fungus Talaromyces sp. SCNU-F0041. Compounds 1 and 2 bear a complicated 6/6/6/5/6/6 hexacyclic ring system characterized by an oxaspiro[5.5]undecane architecture. Compound 3 possesses a benzofuran moiety substituted at C-3 in ergosterol. The structures of the new compounds were identified by comprehensive spectroscopic analysis, X-ray diffraction, and electronic circular dichroism (ECD) calculation. Talarergosteroid B (2) showed significant inhibitory activity against the agricultural plant pathogen Fusarium oxysporum f. sp. lycopersici (MIC = 0.78 μg/mL), outperforming the positive control carbendazim (MIC = 1.56 μg/mL). Preliminary research disclosed that compound 2 may inhibit the spore germination progress, malform the fungal mycelium, and damage the organelle. These results indicate that compound 2 could be a potential fungicidal lead compound against Fusarium oxysporum f. sp. lycopersici.
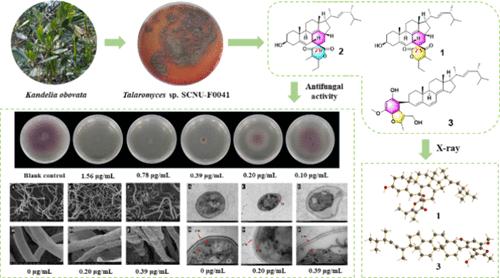
三种具有抗真菌活性的甾体聚酮偶联物
从Kandelia Obovata衍生的真菌Talaromyces sp. scu - f0041中分离到三种先前未被描述的甾体-聚酮缀合物,talar麦角甾a - c(1-3)和talar麦角甾D(4),这是首次从天然来源中鉴定出来的。化合物1和2是一个复杂的6/6/6/5/6/6六环体系,其特征是一个oxaspiro[5.5]十一烷结构。化合物3在麦角甾醇的C-3上具有取代的苯并呋喃部分。通过综合光谱分析、x射线衍射和电子圆二色性(ECD)计算鉴定了新化合物的结构。talarergo甾体B(2)对番茄枯萎病菌(Fusarium oxysporum f. sp. lycopersici)的抑制活性显著(MIC = 0.78 μg/mL),优于阳性对照多菌灵(MIC = 1.56 μg/mL)。初步研究表明,化合物2可能抑制孢子萌发进程,使真菌菌丝畸形,损害细胞器。上述结果表明,化合物2可能是一种潜在的番茄尖孢镰刀菌杀菌剂先导化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


