Constructing High-Yielding Serratia marcescens for (−)-α-Bisabolol Production Based on the Exogenous Haloarchaeal MVA Pathway and Endogenous Molecular Chaperones
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
(−)-α-Bisabolol exhibits analgesic, anti-inflammatory, and skin-soothing properties and is widely applied in the cosmetic and pharmaceutical industries. The use of plant essential oil distillation or chemical synthesis to produce (−)-α-bisabolol is both inefficient and unsustainable. Currently, the microbial production of (−)-α-bisabolol mainly relies on Escherichia coli and Saccharomyces cerevisiae as chassis strains; however, high concentrations of (−)-α-bisabolol have certain toxicity to the strain. This study uses synthetic biology and metabolic engineering strategies to redesign a solvent-tolerant Serratia marcescens for the efficient production of (−)-α-bisabolol. By introducing the Haloarchaea-type mevalonate (MVA) pathway and the (−)-α-bisabolol biosynthesis pathway, we successfully constructed a strain capable of producing (−)-α-bisabolol. The coexpression of the chaperone protein DnaK/J significantly enhanced the soluble expression of the (−)-α-bisabolol synthase, resulting in a 10% increase in (−)-α-bisabolol titer. Furthermore, knockout of the PhoA gene, which reduced the formation of the byproduct farnesol (FOH), further increased the (−)-α-bisabolol titer to 3.21 g/L. In a 5 L bioreactor, S. marcescens achieved a final (−)-α-bisabolol titer of 30.2 g/L, representing the highest titer reported to date. This research provides guidance for the production of (−)-α-bisabolol in nonmodel microorganisms without the requirement for induction.
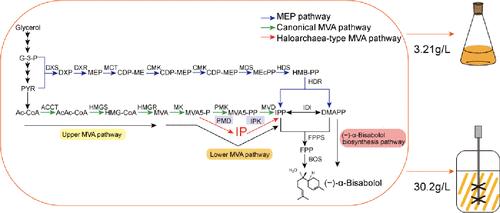
基于外源卤代甲羟戊酸途径和内源分子伴侣蛋白构建高产的 marcescens 沙雷氏菌 (-)-α-Bisabol 生产系统
(-)-α-二十二烷醇具有镇痛、消炎和舒缓皮肤的功效,被广泛应用于化妆品和医药行业。使用植物精油蒸馏法或化学合成法生产 (-)-α- 双羟基苯酚既低效又不可持续。目前,(-)-α-双羟基苯甲酸酯的微生物生产主要以大肠杆菌和酿酒酵母为底物菌株,但高浓度的(-)-α-双羟基苯甲酸酯对菌株有一定的毒性。本研究利用合成生物学和代谢工程策略,重新设计了耐溶剂的 Serratia marcescens,以高效生产(-)-α-bisabol。通过引入半知菌型甲羟戊酸(MVA)途径和(-)-α-双羟基苯酚生物合成途径,我们成功构建了一株能够生产(-)-α-双羟基苯酚的菌株。合子蛋白 DnaK/J 的共表达显著增强了(-)-α-二羟基苯并酚合成酶的可溶性表达,使(-)-α-二羟基苯并酚的滴度增加了 10%。此外,PhoA 基因敲除可减少副产物法尼醇(FOH)的形成,从而进一步将(-)-α-双羟基苯酚滴度提高到 3.21 克/升。在一个 5 升的生物反应器中,S. marcescens 最终获得了 30.2 克/升的(-)-α-双羟基苯酚滴度,这是迄今为止报道的最高滴度。这项研究为在非模式微生物中生产(-)-α-二羟基苯甲酸酚提供了指导,而无需进行诱导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


