Electrochemical Cell for Operando Grazing-Incidence X-ray Absorption Spectroscopic Studies of Low-Loaded Electrodes
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
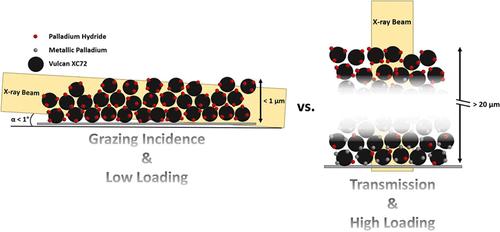
X-ray absorption spectroscopy (XAS) is a powerful technique that provides information about the electronic and local geometric structural properties of newly developed electrocatalysts, especially when it is performed under operating conditions (i.e., operando). However, the large amounts of catalyst typically needed to achieve sufficiently high spectral quality and temporal resolution can result in working electrodes of several micrometers in thickness. This can in turn lead to an inhomogeneous potential distribution across the electrode, delamination, and/or incomplete utilization of the catalyst layer (CL), as well as to the (partial) shielding of the CL with electrochemically evolved bubbles trapped within its pores. These limitations can be tackled by performing such spectrochemical measurements with low-loaded (and thus thin) electrodes, which call for the acquisition of XAS spectra in fluorescence mode and using an X-ray beam incidence angle of ≤0.1° with regards to the working electrode’s substrate plane in a grazing-incidence (GI) configuration. Thus, in this work, we introduce a new spectroelectrochemical flow cell that allows one to perform such measurements in this GI mode and verify its functionality by tracking the potential-induced formation of palladium hydride (PdHx) in a Pd nanoparticle-based electrocatalyst. A time resolution of 10 s per spectrum was achieved with a very low Pd-loading of only 30 μgPd/cm2. Moreover, the implementation of an ion-conductive membrane to separate the working- and counter-electrode compartments enables the quantification of reaction products, which, in the case of gaseous species, can be detected in a time-resolved manner by means of mass spectrometry. Chiefly, this allows us to determine the electrocatalytic activity and selectivity of a given material in the same cell configuration used for the spectroscopic measurements and assures a reliable comparison among the results derived from both techniques.
电化学操作电池-低负荷电极的掠入射x射线吸收光谱研究
X 射线吸收光谱 (XAS) 是一种功能强大的技术,可提供有关新开发的电催化剂的电子和局部几何结构特性的信息,尤其是在工作条件下(即操作)进行时。然而,要获得足够高的光谱质量和时间分辨率,通常需要大量催化剂,这可能导致工作电极的厚度达到几微米。这反过来又会导致整个电极的电位分布不均匀、分层和/或催化剂层(CL)的不完全利用,以及电化学进化的气泡(部分)屏蔽了被困在其孔隙中的催化剂层(CL)。这些限制可通过使用低负载(因此较薄)电极进行光谱化学测量来解决,这就要求在荧光模式下采集 XAS 光谱,并在掠入射 (GI) 配置中使用与工作电极基板平面成 ≤0.1° 角的 X 射线入射角。因此,在这项工作中,我们引入了一种新的光谱电化学流动池,可以在这种 GI 模式下进行此类测量,并通过跟踪基于钯纳米粒子的电催化剂中电位诱导形成的氢化钯(PdHx)来验证其功能。在钯负载量仅为 30 μgPd/cm2 的超低条件下,每个光谱的时间分辨率达到了 10 秒。此外,采用离子导电膜将工作区和反电极区隔开,还能对反应产物进行定量,对于气态产物,可通过质谱仪以时间分辨的方式进行检测。这主要使我们能够在用于光谱测量的相同电池配置中确定特定材料的电催化活性和选择性,并确保对两种技术得出的结果进行可靠的比较。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


