Lactobacillus reuteri-Enriched Eicosatrienoic Acid Regulates Glucose Homeostasis by Promoting GLP-1 Secretion to Protect Intestinal Barrier Integrity
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Lactobacillus reuteri is a well-known probiotic with beneficial effects, such as anti-insulin resistance, anti-inflammatory, and improvement of the intestinal barrier. However, the underlying mechanisms remain unclear. Here, we found that gavage of L. reuteri improved the intestinal barrier and glucose homeostasis in HFD-fed mice. Analysis of lipid metabolomics reveals a significant increase in eicosatrienoic acid (ETA) levels in mouse feces after L. reuteri gavage. We found that ETA maintain intestinal barrier integrity and improve glucose homeostasis by promoting GLP-1 secretion. Mechanistically, by using CD36 inhibitor in vivo and CD36 knockdown STC-1 cells in vitro, we elucidate that ETA activates intestinal CD36-activated PLC/IP3R/Ca2+ signaling to promote GLP-1 secretion. In vivo administration of GLP-1R inhibitor and in vitro intestinal organoid experiments demonstrate that GLP-1 upregulates the PI3K/AKT/HIF-1α pathway by GLP-1R and increases intestinal tight junction protein expressions, which in turn enhance the intestinal barrier integrity, reduce serum LPS level, attenuate inflammation in white adipose tissue (WAT), and ultimately improve glucose homeostasis in HFD and db/db mice. Our study elucidates for the first time the mechanism by which L. reuteri and its enriched metabolite ETA inhibit WAT inflammation by ameliorating the intestinal barrier, ultimately improving glucose homeostasis, and provides a new treatment strategy for T2D.
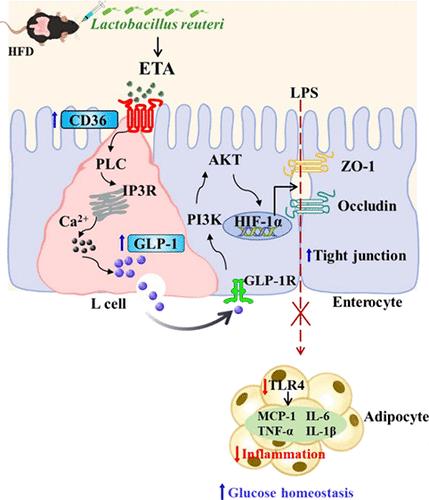
富含罗伊氏乳杆菌的二十碳三烯酸通过促进GLP-1分泌调节葡萄糖稳态,保护肠道屏障完整性
Reuteri 乳杆菌是一种众所周知的益生菌,具有抗胰岛素抵抗、抗炎和改善肠道屏障等有益作用。然而,其潜在机制仍不清楚。在这里,我们发现灌胃 L. reuteri 能改善高纤维食物喂养小鼠的肠道屏障和葡萄糖稳态。脂质代谢组学分析表明,灌胃 L. reuteri 后,小鼠粪便中二十碳三烯酸(ETA)水平显著增加。我们发现 ETA 能维持肠道屏障的完整性,并通过促进 GLP-1 的分泌来改善葡萄糖稳态。通过使用体内 CD36 抑制剂和体外 CD36 敲除 STC-1 细胞,我们从机制上阐明了 ETA 可激活肠道 CD36 激活的 PLC/IP3R/Ca2+ 信号,从而促进 GLP-1 的分泌。体内给予 GLP-1R 抑制剂和体外肠道类器官实验证明,GLP-1 可通过 GLP-1R 上调 PI3K/AKT/HIF-1α 通路,增加肠道紧密连接蛋白的表达,进而增强肠道屏障的完整性,降低血清 LPS 水平,减轻白色脂肪组织(WAT)的炎症反应,最终改善 HFD 和 db/db 小鼠的糖稳态。我们的研究首次阐明了L. reuteri及其富集代谢产物ETA通过改善肠道屏障抑制白脂肪组织炎症反应、最终改善血糖平衡的机制,并为T2D提供了一种新的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.
文献相关原料
公司名称
产品信息
索莱宝
EDTA
索莱宝
protease inhibitor
索莱宝
phosphatase inhibitor
索莱宝
4% paraformaldehyde
索莱宝
penicillin-streptomycin solution
索莱宝
protease inhibitor
索莱宝
4% paraformaldehyde

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


