Effects of salt ions and pH on deamidated soybean protein hydrogels formation: Molecular structure, thermal aggregation and network
IF 8.5
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The aim of this study was to explore the effects of environmental factors (salt ions and pH) on the thermal gelation process of deamidated soy protein isolate (DSPI). The results indicated that with increasing salt ion concentration, DSPI assembled into larger aggregates, which were more prone to aggregation in thermal reactions, ultimately forming a gel network with higher viscoelasticity. The strength enhancement of ion-induced gel networks followed the order from highest to lowest: Ca2+ > Mg2+ > Na+. Regarding pH, as the pH value shifted from 11 to 3, the structure of DSPI transitioned from disorder to order, which promoted protein aggregation, thereby enhancing the strength of the gel network. This work elucidates how environmental factors regulate the gel properties of DSPI through molecular structure, thermal aggregation, and gel networks, thereby laying the foundation for expanding the industrial applications of DSPI.

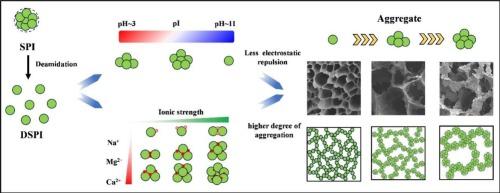
盐离子和 pH 值对脱氨基大豆蛋白水凝胶形成的影响:分子结构、热聚集和网络
本研究旨在探讨环境因素(盐离子和 pH 值)对脱酰胺大豆分离蛋白(DSPI)热凝胶过程的影响。结果表明,随着盐离子浓度的增加,DSPI 聚集成较大的团聚体,在热反应中更容易聚集,最终形成具有较高粘弹性的凝胶网络。离子诱导凝胶网络的强度增强程度从高到低依次为Ca2+ > Mg2+ > Na+。在 pH 值方面,当 pH 值从 11 变为 3 时,DSPI 的结构从无序过渡到有序,这促进了蛋白质的聚集,从而增强了凝胶网络的强度。这项研究阐明了环境因素如何通过分子结构、热聚集和凝胶网络调节DSPI的凝胶特性,从而为扩大DSPI的工业应用奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


