Serpina3n in neonatal microglia mediates its protective role for damaged adult microglia by alleviating extracellular matrix remodeling-induced tunneling nanotubes degradation in a cell model of traumatic brain injury
IF 2.9
3区 医学
Q2 NEUROSCIENCES
引用次数: 0
Abstract
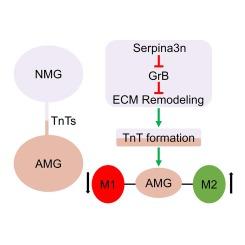
Traumatic brain injury (TBI) induces significant neuroinflammation, primarily driven by microglia. Neonatal microglia (NMG) may have therapeutic potential by modulating the inflammatory response of damaged adult microglia (AMG). This study investigates the influence of NMG on AMG function through extracellular matrix (ECM) remodeling and the formation of tunneling nanotubes (TnTs), with a focus on the role of Serpina3n. We established an in vitro TBI model using a 3D Transwell system, co-culturing damaged AMG with NMG. Viral vector transfection was employed to manipulate Serpina3n expression in NMG. Quantitative real-time PCR, Western blotting, and ELISA were utilized to assess inflammatory markers, ECM remodeling proteins, and TnTs-related proteins. Co-culturing with NMG significantly inhibited M1 polarization of AMG and reduced the release of pro-inflammatory cytokines while promoting M2 polarization and increasing the production of anti-inflammatory cytokines. NMG expressed higher levels of Serpina3n, which played a crucial role in reducing Granzyme B, matrix metalloproteinase (MMP) 2 and MMP9 expression, thereby mitigating ECM remodeling. Inhibition of Serpina3n in NMG increased pro-inflammatory markers and decreased TnTs formation proteins, whereas overexpression of M−sec in AMG counteracted these effects. This highlights the importance of TnTs in maintaining microglial function and promoting an anti-inflammatory environment. In conclusion, NMG improve the function of damaged AMG by modulating ECM remodeling and promoting TnTs formation through the action of Serpina3n.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuroscience
医学-神经科学
CiteScore
6.20
自引率
0.00%
发文量
394
审稿时长
52 days
期刊介绍:
Neuroscience publishes papers describing the results of original research on any aspect of the scientific study of the nervous system. Any paper, however short, will be considered for publication provided that it reports significant, new and carefully confirmed findings with full experimental details.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


