Allergenicity Reduction of Bovine β-Lactoglobulin Binding to Lactic Acid by Masking Epitopes with Lactylation
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
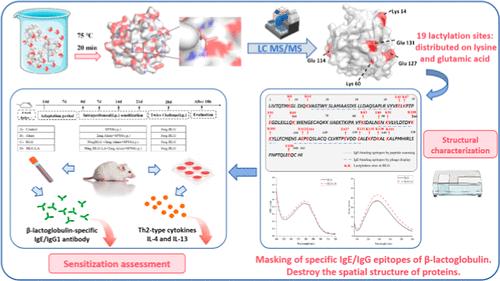
Lactic acid, an important organic acid, commonly exists in a variety of foods. During food processing, lactic acid may undergo dehydration and condensation with proteins. This study investigated the effect of lactylation on the sensitization of bovine β-lactoglobulin during food processing. First, we screened 19 lactylation sites on β-lactoglobulin through mass spectrometry. Comparing the specific IgE/IgG epitopes of β-lactoglobulin, we found that lactylation masks it. At the same time, the structure of β-lactoglobulin is destroyed after binding to lactic acid. Animal experiment results show that the levels of antibodies (IgE and IgG1) and Th2-type cytokines (IL-4 and IL-13) in vivo induced by lactated β-lactoglobulin are significantly reduced. All results indicate that the allergenicity of β-lactoglobulin is reduced after lactylation. In conclusion, this study provides valuable insights into the molecular mechanisms underlying the reduction of β-lactoglobulin allergenicity by lactylation and lays a solid foundation for the application of lactylation in hypoallergenic foods.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


