Therapeutic Monoclonal Antibody─Antidrug Antibody Affinity Constant Determination Using Capillary Electrophoresis–Tandem Mass Spectrometry
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Monoclonal antibody (mAbs) therapeutics cannot evade the occurrence of adverse effects. Thus, mAbs are commonly triggering immune responses corresponding to the expression of antidrug antibodies. Antidrug antibodies can neutralize mAbs, leading to their inhibition and hasten clearance, which dramatically hampers their therapeutic effects. Therefore, studies concerning the affinity between a mAbs and the corresponding antidrug antibody are demonstrating a growing interest to further understand the outcome of biotherapeutics after administration. We describe a novel analytical approach for the determination of the dissociation constant (Kd) between a mAb and an antidrug antibody using capillary electrophoresis coupled to mass spectrometry (CE-MS/MS). The CE-MS/MS method employs a competitive assay, followed by the quantification of the residual amount of the active mAbs. The methodology allowed for the measurement of Kd using serum samples without the implementation of immobilization to achieve protein–protein interaction. This characteristic enabled us to generate the interaction in conditions reflecting the physiological environment. A mathematical treatment was developed to calculate Kd from MS/MS data, taking into account the stoichiometry of the mAbs/antidrug antibody complex and the bivalent properties of the two immunoglobulins. Prior to CE-MS/MS analysis, the interaction of the two proteins was studied by using mass photometry (MP) to determine equilibrium conditions and complexation stoichiometry. CE-MS/MS was used to investigate the interaction between infliximab and a neutralizing anti-infliximab antibody. Results allowed to measure a Kd of 14.4 ± 2.9 nM. MS instrumentation sensitivity and specificity showed to be relevant to achieve accurate and robust Kd measurements for strong interactions in the nanomolar range.
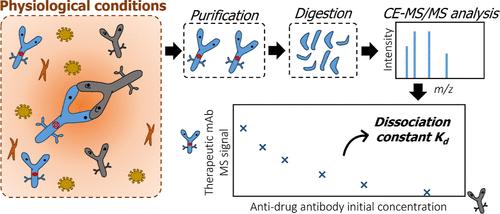
利用毛细管电泳-串联质谱法测定治疗用单克隆抗体-抗药性抗体亲和力常数
单克隆抗体(mAbs)疗法无法避免不良反应的发生。因此,mAbs 通常会引发与抗药抗体表达相对应的免疫反应。抗药抗体可以中和 mAbs,导致抑制和加速清除,从而极大地影响其治疗效果。因此,有关 mAbs 与相应抗药抗体之间亲和力的研究越来越受到人们的关注,以进一步了解生物治疗药物给药后的结果。我们介绍了一种利用毛细管电泳耦合质谱(CE-MS/MS)测定 mAb 与抗药抗体之间解离常数(Kd)的新型分析方法。CE-MS/MS 方法采用竞争性测定,然后对活性 mAb 的残留量进行定量。该方法允许使用血清样本测量 Kd,而无需通过固定来实现蛋白质之间的相互作用。这一特点使我们能够在反映生理环境的条件下产生相互作用。考虑到 mAbs/抗药抗体复合物的化学计量学和两种免疫球蛋白的双价特性,我们开发了一种数学处理方法来计算 MS/MS 数据中的 Kd。在进行 CE-MS/MS 分析之前,利用质量光度法(MP)研究了两种蛋白质的相互作用,以确定平衡条件和复合物的化学计量学。CE-MS/MS 用于研究英夫利昔单抗与中和抗英夫利昔单抗抗体之间的相互作用。结果测得 Kd 为 14.4 ± 2.9 nM。MS 仪器的灵敏度和特异性表明,对于纳摩尔范围内的强相互作用,Kd 测量的准确性和稳健性非常重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


