Utilizing the carbon nano-belt (8-CNB) loaded late second-row transition metal (TM) single-atom catalysts for hydrogen and oxygen evolution during water electrolysis
IF 4.2
3区 工程技术
Q2 ENGINEERING, ELECTRICAL & ELECTRONIC
引用次数: 0
Abstract
Production of high purity of hydrogen and oxygen via water electrolysis is setback by the high overpotential associated with water splitting. In this regard, herein the use of late second-row transition metal (TM) doped carbon nano-belt (8-CNB) as single atom catalysts for water electrolysis is investigated via density functional theory (DFT) calculations. The unique unsaturated belt-shaped structure of 8-CNB introduces functional sites that are suitable for TM anchoring. As such, the designed catalysts have shown high stability. The high stability was found to originate from the chemisorption of the metals to the support, as confirmed by the quantum theory of atoms in molecules (QTAIM) analysis. Moreover, the doped structures have shown low frontier molecular orbitals gap (HOMO-LUMO Egap) values, indicating sufficient electrical conductivities, which is desirable in water electrolysis to facilitate the transfer of electrons. Furthermore, the catalytic activity results have shown that the Ru@8-CNB and Rh@8-CNB systems are highly active towards the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), respectively. Results have shown that Ru@8-CNB exhibits a low ΔGH value of 0.12 eV towards the HER, while Rh@8-CNB revealed a low overpotential value of 0.63 V towards the OER. The proposed SACs have catalytic activities that are competitive to the highly active Pt(III) catalyst and they are advantageous in their high atom percentage efficiency as SACs.
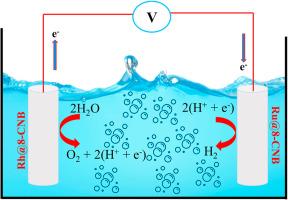
利用碳纳米带(8-CNB)负载的晚期第二排过渡金属(TM)单原子催化剂在电解水过程中进行氢氧演化
通过电解水来生产高纯度的氢气和氧气,会因水分子分裂时产生的高过电位而受到影响。为此,本文通过密度泛函理论(DFT)计算,研究了晚期第二排过渡金属(TM)掺杂碳纳米带(8-CNB)作为单原子催化剂用于水电解的情况。8-CNB 独特的不饱和带状结构引入了适合 TM 固定的功能位点。因此,所设计的催化剂具有很高的稳定性。分子中原子量子理论(QTAIM)分析证实,这种高稳定性源于金属对载体的化学吸附作用。此外,掺杂结构显示出较低的前沿分子轨道间隙(HOMO-LUMO Egap)值,表明具有足够的导电性,这在水电解中有利于电子的转移。此外,催化活性结果表明,Ru@8-CNB 和 Rh@8-CNB 系统分别对氢进化反应(HER)和氧进化反应(OER)具有很高的活性。结果表明,Ru@8-CNB 在氢进化反应中表现出 0.12 eV 的低 ΔGH 值,而 Rh@8-CNB 在氧进化反应中表现出 0.63 V 的低过电位值。所提出的 SAC 具有与高活性铂(III)催化剂竞争的催化活性,而且它们作为 SAC 具有原子百分比效率高的优势。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Science in Semiconductor Processing
工程技术-材料科学:综合
CiteScore
8.00
自引率
4.90%
发文量
780
审稿时长
42 days
期刊介绍:
Materials Science in Semiconductor Processing provides a unique forum for the discussion of novel processing, applications and theoretical studies of functional materials and devices for (opto)electronics, sensors, detectors, biotechnology and green energy.
Each issue will aim to provide a snapshot of current insights, new achievements, breakthroughs and future trends in such diverse fields as microelectronics, energy conversion and storage, communications, biotechnology, (photo)catalysis, nano- and thin-film technology, hybrid and composite materials, chemical processing, vapor-phase deposition, device fabrication, and modelling, which are the backbone of advanced semiconductor processing and applications.
Coverage will include: advanced lithography for submicron devices; etching and related topics; ion implantation; damage evolution and related issues; plasma and thermal CVD; rapid thermal processing; advanced metallization and interconnect schemes; thin dielectric layers, oxidation; sol-gel processing; chemical bath and (electro)chemical deposition; compound semiconductor processing; new non-oxide materials and their applications; (macro)molecular and hybrid materials; molecular dynamics, ab-initio methods, Monte Carlo, etc.; new materials and processes for discrete and integrated circuits; magnetic materials and spintronics; heterostructures and quantum devices; engineering of the electrical and optical properties of semiconductors; crystal growth mechanisms; reliability, defect density, intrinsic impurities and defects.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


