Tea Polyphenols Reduced Obesity by Modulating Gut Microbiota-SCFAs-Barrier and Inflammation in High-Fat Diet-Induced Mice
Abstract
Scope
Obesity by high-fat diets (HFDs) is a chronic metabolic disorder that poses a significant threat to human health. Tea polyphenols (TPs) can prevent obesity caused by HFD by modulating gut microbiota.
Methods and results
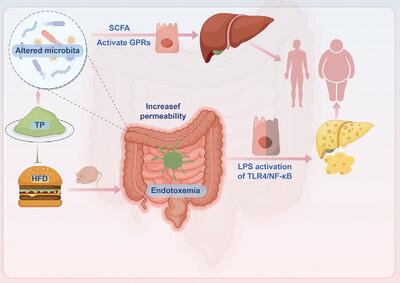
To explore the function of TP in mitigating the effects of obesity and inflammation, mice are fed HFDs either with or without TP. TP supplementation effectively attenuates HFD-induced weight gain, liver and adipose tissue accumulation, while also improving liver fat content as well as colon and ileum tissue morphology. TP supplementation leads to a downregulation of lipid accumulation genes and an upregulation of lipid-decomposition genes. Moreover, TP increases Blautia and Faecalibaculum while reducing the Colidextribacter and short-chain fatty acids in HFD-induced mice, significantly activates G protein-coupled receptors, inhibits histone deacetylases, enhances intestinal tight junction expression levels, reduces intestinal permeability, and thereby preserves intestinal barrier integrity. Additionally, TP markedly suppresses the expression of inflammatory cytokines and inhibits the activation of TLR4 signaling pathways.
Conclusion
These findings suggest that TP holds great promise for improving both obesity management and alleviating intestinal inflammation, and provides a clue for understanding the antiobesity effects of TP.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


