Modification of Azo-Aminopyrimidines as Potent Multitarget Inhibitors of Insect Chitinolytic Enzymes OfChi-h and OfHex1
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Multitarget inhibitors exhibit significant advantages in reducing the risk of drug resistance, enhancing therapeutic efficacy, and minimizing dosage, outperforming multicomponent combination drugs. On the basis of glycoside hydrolase family 18 (GH18) chitinases and GH20 β-N-acetylhexosaminidase using the same substrate-assisted catalytic mechanism and similar substrate binding modes, a series of novel azo-aminopyrimidine compounds have been designed and synthesized as multitarget inhibitors targeting chitinolytic enzymes OfChi-h and OfHex1. Compounds AAP4 (OfChi-h, Ki = 29.3 nM; OfHex1, Ki = 4.9 μM) and AAP16 (OfChi-h, Ki = 32.4 nM; OfHex1, Ki = 7.2 μM) were identified to be potent multitarget inhibitors of these enzymes, which were predicted to occupy the −1 subsite and engage in H-binding interactions with catalytic residues. AAP4 also displayed significant insecticidal activity against lepidopteran pests Ostrinia furnacalis through leaf dipping and pot experiments. In addition, the safety of AAP4 to corn and the natural enemy Trichogramma ostriniae was comprehensively evaluated. This present work indicates that azo-aminopyrimidines, as multitarget inhibitors against chitinolytic enzymes, can be further developed as safe and efficient pest control and management agents.
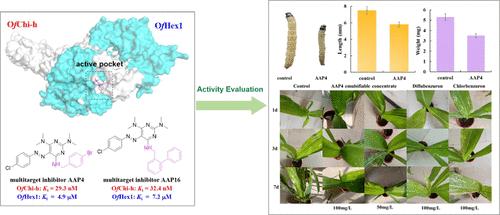
修饰偶氮氨基嘧啶作为昆虫几丁质分解酶 OfChi-h 和 OfHex1 的强效多靶点抑制剂
多靶点抑制剂在降低耐药性风险、提高疗效和减少用量方面具有显著优势,优于多组分复方药物。基于糖苷水解酶家族 18(GH18)几丁质酶和 GH20 β-N-乙酰己糖胺酶具有相同的底物辅助催化机理和相似的底物结合模式,我们设计并合成了一系列新型偶氮氨基嘧啶化合物,作为针对几丁质溶解酶 OfChi-h 和 OfHex1 的多靶点抑制剂。经鉴定,化合物 AAP4(OfChi-h,Ki = 29.3 nM;OfHex1,Ki = 4.9 μM)和 AAP16(OfChi-h,Ki = 32.4 nM;OfHex1,Ki = 7.2 μM)是这些酶的强效多靶点抑制剂,据预测,它们会占据-1 亚位点,并与催化残基发生 H 结合相互作用。通过叶片浸渍和盆栽实验,AAP4 对鳞翅目害虫 Ostrinia furnacalis 也显示出显著的杀虫活性。此外,还全面评估了 AAP4 对玉米和天敌旋毛虫的安全性。本研究结果表明,偶氮氨基嘧啶类化合物作为几丁质分解酶的多靶点抑制剂,可进一步开发为安全高效的害虫防治剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


