Unraveling distinct potential of pea (Pisum sativum L.) fractions (legumin, vicilin and albumin) by structural and functional characterization
IF 7
1区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
Limited and unclear research exists on the individual capacity of major fractions of pea protein legumin (PL), vicilin (PV) and albumin (PA), which collectively contribute to the structural and functional properties of pea protein. Findings revealed that PV (72.26 ± 2.6 %) and PA (57.42 ± 4.1 %) displayed better solubility compared to PL. PL fraction possessed a complex three-dimensional structure, higher surface hydrophobicity (So), and superior oil-holding-capacity (OHC) contributing to its 4-fold strength (8.58 ± 0.5 N) and structured gel formation. The smaller particle size of PA was also accountable for the comparatively weaker gels and unstable emulsions compared to PL, while PV had the least emulsifying capacity, by non-uniform droplet distribution in CLSM served as proof. PL was found to be responsible for gelation, emulsification, and foaming in pea protein due to structural factors (relative abundance of α-helix and β-sheet). While, the flexible structure of PV, absence of cysteine residues, and disulfide bridges played a role in characteristics like foaming stability. Some protein in PV gel was found loose and did not appear to participate in gelation, hence forming a significantly weaker gel than PL. Despite relatively less So and complex structure, albumin (PA) had a smoother but weaker gel, more consistent and a smaller droplet size distribution in emulsions (compared to PV). Nonetheless, this study aims to fill a forgotten gap by providing baseline knowledge on the individual fractions of pea protein, defining their roles and paving the path for future research focusing on structural and functional properties of pea protein.
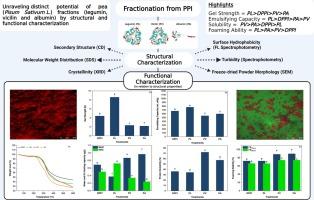
通过结构和功能表征揭示豌豆(Pisum sativum L.)组分(豆蛋白、vicilin 和白蛋白)的独特潜力
关于豌豆蛋白的主要成分豆胶(PL)、麦角蛋白(PV)和白蛋白(PA)各自能力的研究有限且不明确,这些成分共同构成了豌豆蛋白的结构和功能特性。研究结果表明,PV(72.26 ± 2.6 %)和 PA(57.42 ± 4.1 %)的溶解度优于 PL。PL 部分具有复杂的三维结构、更高的表面疏水性(So)和更优越的持油能力(OHC),使其强度增加了 4 倍(8.58 ± 0.5 N),并形成了结构凝胶。与 PL 相比,PA 的粒径较小,因此凝胶较弱,乳液不稳定,而 PV 的乳化能力最低,CLSM 中液滴分布不均匀就是证明。由于结构因素(α-螺旋和β-片的相对丰度),PL 对豌豆蛋白的凝胶化、乳化和起泡起作用。而豌豆蛋白的柔性结构、半胱氨酸残基和二硫桥的缺失则对发泡稳定性等特性产生了影响。在 PV 凝胶中发现一些蛋白质比较松散,似乎没有参与凝胶化,因此形成的凝胶比 PL 弱得多。尽管白蛋白(PA)的So相对较少且结构复杂,但与PV相比,白蛋白(PA)的凝胶更光滑但更弱,在乳液中更稳定且液滴大小分布更小。尽管如此,这项研究旨在填补一个被遗忘的空白,提供有关豌豆蛋白各个组分的基本知识,确定它们的作用,并为今后重点研究豌豆蛋白的结构和功能特性铺平道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Research International
工程技术-食品科技
CiteScore
12.50
自引率
7.40%
发文量
1183
审稿时长
79 days
期刊介绍:
Food Research International serves as a rapid dissemination platform for significant and impactful research in food science, technology, engineering, and nutrition. The journal focuses on publishing novel, high-quality, and high-impact review papers, original research papers, and letters to the editors across various disciplines in the science and technology of food. Additionally, it follows a policy of publishing special issues on topical and emergent subjects in food research or related areas. Selected, peer-reviewed papers from scientific meetings, workshops, and conferences on the science, technology, and engineering of foods are also featured in special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


