Myosin supramolecular self-assembly: The crucial precursor that manipulates the covalent aggregation, emulsification and rheological properties of myosin
IF 7
1区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
The transformation of molecular conformation and self-assembly properties of myosin during the heating process at different ionic strengths (0.2 M, 0.4 M and 0.6 M NaCl) and its effect on rheological behavior and emulsification properties were investigated. Under incubation temperatures between 40 °C and 50 °C, myosin underwent a supramolecular self-assembly stage dominated by noncovalent forces (hydrogen bonding, ionic bonding and hydrophobic interactions). Higher ionic strength facilitated molecular rearrangement through enhanced swelling of myosin heads and head-to-head assemblies, which contributed to enhanced ordering and homogeneity of myosin covalent aggregates (above 60 °C) and manifested itself macroscopically as enhanced gel viscoelasticity and emulsion stability. In contrast, at lower ionic strength, the tail-to-tail assemblies of myosin led to the preferential formation of covalent cross-links in the tails, which resulted in the inability of molecular rearrangement and the formation of disordered aggregates and finally led to the deterioration of the gel and the destabilization of the emulsion. In conclusion, the supramolecular self-assembly behavior of myosin, as an intermediate process in myosin’s sol–gel transition, is crucial for the orderliness of myosin assemblies, gel network strengthening, and emulsion stability. The obtained insight provides a reference for the precise implementation of quality improvement strategies for meat products.
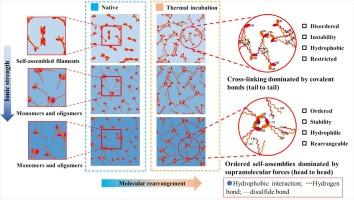
肌球蛋白超分子自组装:操纵肌球蛋白共价聚集、乳化和流变特性的重要前体
研究了肌球蛋白在不同离子强度(0.2 M、0.4 M 和 0.6 M NaCl)下加热过程中分子构象和自组装特性的变化及其对流变行为和乳化特性的影响。在 40 °C 至 50 °C 的培养温度下,肌球蛋白经历了一个由非共价作用力(氢键、离子键和疏水相互作用)主导的超分子自组装阶段。较高的离子强度通过增强肌球蛋白头部的膨胀和头对头的组装促进了分子的重新排列,这有助于增强肌球蛋白共价聚集体的有序性和均匀性(60 °C以上),并在宏观上表现为凝胶粘弹性和乳液稳定性的增强。相反,在较低的离子强度下,肌球蛋白尾端到尾端的组装导致尾端优先形成共价交联,从而导致分子无法重新排列,形成无序的聚集体,最终导致凝胶恶化和乳液不稳定。总之,肌球蛋白的超分子自组装行为是肌球蛋白溶胶-凝胶转变的中间过程,对肌球蛋白组装的有序性、凝胶网络的强化和乳液的稳定性至关重要。这一发现为精确实施肉制品质量改进策略提供了参考。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Research International
工程技术-食品科技
CiteScore
12.50
自引率
7.40%
发文量
1183
审稿时长
79 days
期刊介绍:
Food Research International serves as a rapid dissemination platform for significant and impactful research in food science, technology, engineering, and nutrition. The journal focuses on publishing novel, high-quality, and high-impact review papers, original research papers, and letters to the editors across various disciplines in the science and technology of food. Additionally, it follows a policy of publishing special issues on topical and emergent subjects in food research or related areas. Selected, peer-reviewed papers from scientific meetings, workshops, and conferences on the science, technology, and engineering of foods are also featured in special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


