Molten globule-state protein structure: Perspectives from food processing applications
IF 7
1区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
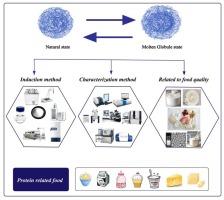
Under specific pretreatment or processing conditions, spheroprotein can be transformed into a molten globule state, a typical protein conformation with enhanced functionality. Analyzing the correlation between the formation of molten-globule structures and their quality and functional characteristics is critical for developing tailored processing features, especially for minimally processed future foods. This review outlines the mechanisms driving the formation of molten globule proteins through various processes including ultra-high pressure pretreatments, heating, ultrasonication, pH-shifting, macromolecular crowding and exposure to small-molecule denaturants. These treatments yield proteins that retain structural compactness and primary and secondary structures of their native forms, but with modified conformations and increased hydrophobicity. Common methods for characterizing molten globule proteins include fluorescence spectroscopy, circular dichroism spectroscopy, and nuclear magnetic resonance. The review also explores the application of molten globule proteins in food processing, highlighting their potential significance in advancing the field. The detailed elucidation and exploration of the microstructural transition and conformational features of molten globule proteins, together with their quantitative relationship with processibility of proteins from various sources, holds significant implications for optimizing protein-based food processing techniques and achieving targeted improvements in food quality.
熔融球状蛋白质结构:食品加工应用的视角
在特定的预处理或加工条件下,球蛋白可转变为熔融球状,这是一种具有增强功能的典型蛋白质构象。分析熔融球状结构的形成与其质量和功能特性之间的相关性,对于开发定制加工功能,尤其是未来的微加工食品至关重要。本综述概述了通过超高压预处理、加热、超声波、pH 值变化、大分子排挤和接触小分子变性剂等各种工艺形成熔融球状蛋白质的机理。经过这些处理后,蛋白质的结构紧密性、一级和二级结构都保持了原生形态,但构象发生了改变,疏水性增强。表征熔融球蛋白的常用方法包括荧光光谱法、圆二色光谱法和核磁共振法。综述还探讨了熔融球蛋白在食品加工中的应用,强调了其在推动该领域发展方面的潜在意义。详细阐明和探索熔融球蛋白的微观结构转变和构象特征,以及它们与各种来源蛋白质加工性的定量关系,对优化基于蛋白质的食品加工技术和实现有针对性的食品质量改善具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Research International
工程技术-食品科技
CiteScore
12.50
自引率
7.40%
发文量
1183
审稿时长
79 days
期刊介绍:
Food Research International serves as a rapid dissemination platform for significant and impactful research in food science, technology, engineering, and nutrition. The journal focuses on publishing novel, high-quality, and high-impact review papers, original research papers, and letters to the editors across various disciplines in the science and technology of food. Additionally, it follows a policy of publishing special issues on topical and emergent subjects in food research or related areas. Selected, peer-reviewed papers from scientific meetings, workshops, and conferences on the science, technology, and engineering of foods are also featured in special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


