Isolation of ice structuring proteins from winter wheat in frigid region (Dongnongdongmai1) and the effect on freeze–thaw stability of dough
IF 7
1区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
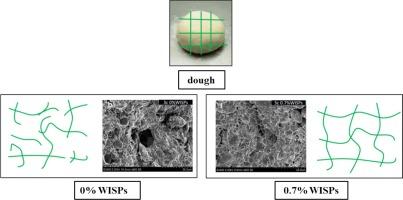
In this study, ice structuring proteins (WISPs) extracted from winter wheat in a frigid region were prepared and added to frozen-thawed dough. The WISPs were characterized, revealing that they contained a higher proportion of hydrophilic amino acids and had a molecular weight of approximately 15 kDa. The highest thermal hysteresis activity (THA) observed was 0.62 °C. The secondary structure of WISPs was determined to be as follows: β-sheet: 49.33 %, random coil: 13.87 %, α-helix: 16.35 %, β-turn: 20.45 %. The study investigated the effects of different additions of WISPs on the water mobility, glass transition temperature, microstructure, rheological properties, and texture analysis of frozen-thawed dough. The results demonstrated that the inclusion of WISPs reduced the fluidity of water and water migration in the dough during the frozen-thawed cycle. This protective effect preserved the internal structure and gluten network of the dough, leading to increased viscosity, elasticity, and improved texture properties of the frozen-thawed dough. Furthermore, the addition of WISPs at concentrations ranging from 0 % to 0.7 % resulted in a 1.8 °C increase in the glass transition temperature (Tg). Overall, these findings suggest that WISPs can serve as a beneficial additive for enhancing the freeze–thaw stability of dough.
从寒带冬小麦(冬农冬麦1)中分离冰结构蛋白及其对面团冻融稳定性的影响
本研究制备了从寒冷地区冬小麦中提取的冰结构蛋白(WISPs),并将其添加到冷冻解冻的面团中。对 WISPs 进行了表征,发现它们含有较高比例的亲水性氨基酸,分子量约为 15 kDa。观察到的最高热滞后活性(THA)为 0.62 °C。经测定,WISPs 的二级结构如下:β-片状结构:49.33 %,随机线圈13.87 %,α-螺旋:16.35 %,β-匝:20.45 %。该研究探讨了不同 WISPs 添加量对冻融面团的水迁移率、玻璃化转变温度、微观结构、流变特性和质构分析的影响。结果表明,在冷冻-解冻周期中,WISPs 的加入降低了面团中水的流动性和水的迁移。这种保护作用保留了面团的内部结构和面筋网络,从而提高了冻融面团的粘度和弹性,并改善了其质地特性。此外,添加浓度为 0 % 至 0.7 % 的 WISPs 可使玻璃化转变温度(Tg)提高 1.8 °C。总之,这些研究结果表明,WISPs 可以作为一种有益的添加剂来提高面团的冻融稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Research International
工程技术-食品科技
CiteScore
12.50
自引率
7.40%
发文量
1183
审稿时长
79 days
期刊介绍:
Food Research International serves as a rapid dissemination platform for significant and impactful research in food science, technology, engineering, and nutrition. The journal focuses on publishing novel, high-quality, and high-impact review papers, original research papers, and letters to the editors across various disciplines in the science and technology of food. Additionally, it follows a policy of publishing special issues on topical and emergent subjects in food research or related areas. Selected, peer-reviewed papers from scientific meetings, workshops, and conferences on the science, technology, and engineering of foods are also featured in special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


