Compartmentalization vs. segregation of reactants: Accomplishment of the Maillard reaction at the water-water interface
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
All-aqueous (water-in-water) emulsions are increasingly used as droplets reactors. The present communication reports that precursors of a reaction segregated by partitioning between emulsion phases can undergo reaction at the interface, i.e., on droplet surface, while the interface remains liquid. Na2SO4-in-polyethylene glycol (PEG) emulsions were prepared, and precursors (glucose, asparagine, and tryptophan) of the Maillard reaction were partitioned either inside the droplets (co-encapsulation) or segregated between the emulsion interior and exterior phases. It was found that following the interfacial (i.e., on-droplet) reaction of the segregated precursors, 99 % of the Amadori product N-(1-deoxy-D-fructos-1-yl)-L-tryptophan (Fru-Trp) partitioned into the PEG phase. Also, hydrophobic advanced reaction products including β-carboline derivatives and Strecker aldehyde, alongside melanoidins, showed a clear affinity towards the PEG phase. Once the precursors were co-encapsulated within Na2SO4 droplets, following their generation succinimide and pyridine derivatives remained partitioned within the droplets, whereas N-hydroxysuccinimide, pyrrole derivatives, and melanoidins predominantly partitioned into the PEG phase.
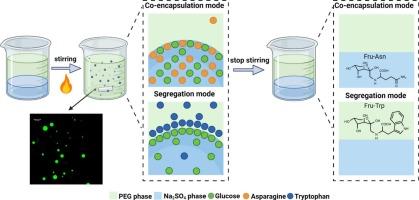
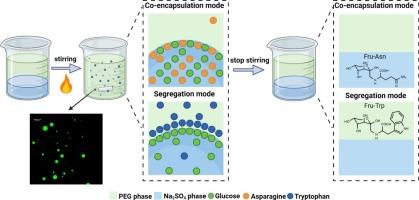
反应物的分隔与隔离:在水-水界面上完成马氏反应
全水(水包水)乳液越来越多地被用作液滴反应器。本研究报告指出,通过乳液相之间的分隔而分离的反应前体可以在界面(即液滴表面)上发生反应,而界面仍保持液态。制备了聚乙二醇(PEG)乳液中的 Na2SO4,并将 Maillard 反应的前体(葡萄糖、天冬酰胺和色氨酸)分隔在液滴内部(共包囊)或乳液内部和外部相之间。研究发现,在分离的前体发生界面(即液滴上)反应后,约 99% 的 Amadori 产物 N-(1-脱氧-D-果糖-1-基)-L-色氨酸(Fru-Trp)被分隔到 PEG 相中。此外,包括 β-咔啉衍生物和 Strecker 醛在内的疏水性高级反应产物以及类黑色素也对 PEG 相表现出明显的亲和力。前体在 Na2SO4 液滴中共同封装后,生成的琥珀酰亚胺和吡啶衍生物仍留在液滴中,而 N-羟基琥珀酰亚胺、吡咯衍生物和麦拉宁则主要进入 PEG 相。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


