Central treatment of neuropeptide-S attenuates cognitive dysfunction and hippocampal synaptic plasticity impairment by increasing CaMKII/GluR1 in hemiparkinsonian rats
IF 2.9
3区 医学
Q2 NEUROSCIENCES
引用次数: 0
Abstract
Neuropeptide-S (NPS) has been demonstrated to mitigate learning and memory deficits in experimental models of Parkinson’s Disease (PD). Despite this, the precise mechanisms through which NPS exerts its influence on cognitive functions remain to be fully unknown. This study aims to elucidate the effects of central administration of NPS on learning and memory deficits associated with an experimental rat hemiparkinsonian model, examining both electrophysiological and molecular parameters. The hemiparkinsonian model was established via stereotactic injection of 6-hydroxydopamine (6-OHDA) into the right medial forebrain bundle. Central NPS (1 nmol, icv) was administered into the lateral ventricle via a cannula for seven consecutive days following the 6-OHDA lesion. The Morris water maze and object recognition tests were used to evaluate the rat’s learning and memory abilities. Long-term potentiation (LTP) recordings were conducted to assess hippocampal synaptic plasticity. Immunohistochemistry was employed to determine the expression levels of phosphorylated CaMKII (pCaMKII), GluR1, and GluR2 in the hippocampus. The 6-OHDA-induced decline in cognitive performance was significantly (p < 0.05) improved in rats that received central NPS. In 6-OHDA-lesioned rats, NPS treatment significantly (p < 0.05) enhanced the amplitude of LTP at the dentate gyrus/perforant path synapses. Furthermore, NPS significantly (p < 0.05) increased the number of pCaMKII and GluR1 immunoreactive cells in the hippocampus, which had been diminished due to 6-OHDA, except for GluR2 levels. These findings provide insight into the mechanisms by which central NPS administration enhances cognitive functions in an experimental model of PD, highlighting its potential therapeutic benefits for addressing cognitive deficits in PD.
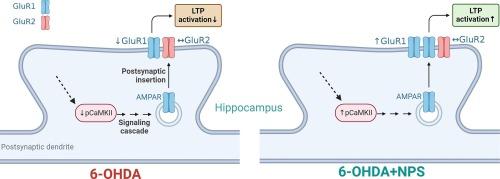
通过增加CaMKII/GluR1,中枢治疗神经肽-S可减轻半帕金森大鼠的认知功能障碍和海马突触可塑性损伤。
神经肽-S(NPS)已被证明可以减轻帕金森病(PD)实验模型中的学习和记忆缺陷。尽管如此,NPS对认知功能产生影响的确切机制仍然完全未知。本研究旨在阐明中枢给药 NPS 对与实验性大鼠半帕金森病模型相关的学习和记忆障碍的影响,同时检查电生理学和分子参数。半帕金森病模型是通过向右侧内侧前脑束立体定向注射 6-羟基多巴胺(6-OHDA)建立的。在6-OHDA病变后连续七天通过插管向侧脑室注射中枢NPS(1 nmol,icv)。莫里斯水迷宫和物体识别测试用于评估大鼠的学习和记忆能力。长期电位(LTP)记录用于评估海马突触可塑性。免疫组化法测定了磷酸化 CaMKII(pCaMKII)、GluR1 和 GluR2 在海马中的表达水平。6-OHDA诱导的认知能力下降显著(p
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuroscience
医学-神经科学
CiteScore
6.20
自引率
0.00%
发文量
394
审稿时长
52 days
期刊介绍:
Neuroscience publishes papers describing the results of original research on any aspect of the scientific study of the nervous system. Any paper, however short, will be considered for publication provided that it reports significant, new and carefully confirmed findings with full experimental details.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


